Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis
- PMID: 24874116
- PMCID: PMC4317408
- DOI: 10.1007/s11999-014-3699-2
Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis
Abstract
Background: Modular megaprostheses are now the most common method of reconstruction after segmental resection of the long bones in the lower extremities. Previous studies reported variable outcome and failure rates after knee megaprosthetic reconstructions.
Questions/purposes: The objectives of this study were to analyze the results of a modular tumor prosthesis after resection of bone tumor around the knee with respect to (1) survivorship; (2) failure rate; (3) comparative survivorship against different sites of reconstructions and of primary and revision implants; and (4) functional results on the Musculoskeletal Tumor Society (MSTS) scoring system.
Methods: Between 2003 and 2010, 247 rotating-hinge Global Modular Reconstruction System (GMRS) knee prostheses were implanted in our institute for malignant and aggressive benign tumors. During this time, that group represented 23% of the patients who had oncologic megaprosthesis reconstruction about the knee after resection of primary or metastatic bone tumors (247 of 1086 patients). In the other 77% of cases we used other types of oncologic prostheses. Before 2003 we used the older Howmedica Modular Resection System and Kotz Modular Femur/Tibia Replacement from 2003 we used mostly the GMRS but we continued to use the HMRS in some cases such as patients with poor prognoses, elderly patients, or metastatic patients. Sites included 187 distal femurs and 60 proximal tibias. Causes of megaprosthesis failure were classified according to Henderson et al. in five types: Type 1 (soft tissue failure), Type 2 (aseptic loosening), Type 3 (structural failure), Type 4 (infection), and Type 5 (tumor progression). Followup was at a minimum oncologic followup of 2 years (mean, 4 years; range, 2-8 years). Kaplan-Meier actuarial curves of implant survival to major failures were done. Functional results were analyzed according to the MSTS II system; 223 of the 247 were available for functional scoring (81%).
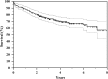
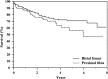
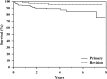
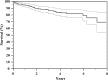
Results: At latest followup, among 175 treated patients for primary reconstruction, 117 are continuously disease-free, 26 have no evidence of disease after treatment of relapse, eight are alive with disease, and 24 died from disease. The overall failure rate of the megaprostheses in our series was 29.1% (72 of 247). Type 1 failure occurred in 8.5% (21 of 247) cases, Type 2 in 5.6% (14 of 247), Type 3 in 0%, Type 4 in 9.3% (23 of 247), and Type 5 in 5.6% (14 of 247). Kaplan-Meier curve showed an overall implant survival rate for all types of failures of 70% at 4 years and 58% at 8 years. Prosthetic survivorship for revisions was 80% at 5 years and for primary reconstructions was 60% at 5 years (p = 0.013). Survivorship to infection was 95% at 5 years for revision patients and 84% at 5 years for primary patients (p = 0.475). The mean MSTS score was 84 (25.2; range, 8-30) with no difference between sites of localization (24.7 in proximal tibia versus 25.4 in distal femur reconstruction; p = 0.306).
Conclusions: Results at a minimum of 2 years with this modular prosthesis are satisfactory in terms of survivorship (both oncologic and reconstructive) and causes and rates of failure. Although these results seem comparable with other like implants, we will continue to follow this cohort, and we believe that comparative trials among the available megaprosthesis designs are called for.
Level of evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Figures

References
-
- Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M, Campanacci M. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–4027. - PubMed
-
- Bacci G, Picci P, Ferrari S, Avella M, Prever BA, Ruggieri P, Casadei R, Lari S, Monti C, Cazzola A. Neoadjuvant chemotherapy for nonmetastatic osteosarcoma for the extremities: the recent experience at the Rizzoli Institute. Cancer Treat Res. 1993;62:299–308. doi: 10.1007/978-1-4615-3518-8_36. - DOI - PubMed
-
- Bacci G, Picci P, Ferrari S, Ruggieri P, Casadei R, Tienghi A, Brach del Prever A, Gherlinzoni F, Mercuri M, Monti C. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–3238. doi: 10.1002/1097-0142(19931201)72:11<3227::AID-CNCR2820721116>3.0.CO;2-C. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

