Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain
- PMID: 24874470
- PMCID: PMC6483540
- DOI: 10.1002/14651858.CD011056.pub2
Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain
Abstract
Background: There is increasing focus on providing high quality care for people at the end of life, irrespective of disease or cause, and in all settings. In the last ten years the use of care pathways to aid those treating patients at the end of life has become common worldwide. The use of the Liverpool Care Pathway in the UK has been criticised. In England the LCP was the subject of an independent review, commissioned by a Health Minister. The Neuberger Review acknowledged that the LCP was based on the sound ethical principles that provide the basis of good quality care for patients and families when implemented properly. It also found that the LCP often was not implemented properly, and had instead become a barrier to good care; it made over 40 recommendations, including education and training, research and development, access to specialist palliative care services, and the need to ensure care and compassion for all dying patients. In July 2013, the Department of Health released a statement that stated the use of the LCP should be "phased out over the next 6-12 months and replaced with an individual approach to end of life care for each patient".The impact of opioids was a particular concern because of their potential influence on consciousness, appetite and thirst in people near the end of life. There was concern that impaired patient consciousness may lead to an earlier death, and that effects of opioids on appetite and thirst may result in unnecessary suffering. This rapid review, commissioned by the National Institute for Health Research, used standard Cochrane methodology to examine adverse effects of morphine, fentanyl, oxycodone, and codeine in cancer pain studies as a close approximation to possible effects in the dying patient.
Objectives: To determine the impact of opioid treatment on patient consciousness, appetite and thirst in randomised controlled trials of morphine, fentanyl, oxycodone or codeine for treating cancer pain.
Search methods: We assessed adverse event data reported in studies included in current Cochrane reviews of opioids for cancer pain: specifically morphine, fentanyl, oxycodone, and codeine.
Selection criteria: We included randomised studies using multiple doses of four opioid drugs (morphine, fentanyl, oxycodone, and codeine) in cancer pain. These were taken from four existing or ongoing Cochrane reviews. Participants were adults aged 18 and over. We included only full journal publication articles.
Data collection and analysis: Two review authors independently extracted adverse event data, and examined issues of study quality. The primary outcomes sought were numbers of participants experiencing adverse events of reduced consciousness, appetite, and thirst. Secondary outcomes were possible surrogate measures of the primary outcomes: delirium, dizziness, hallucinations, mood change and somnolence relating to patient consciousness, and nausea, vomiting, constipation, diarrhoea, dyspepsia, dysphagia, anorexia, asthenia, dehydration, or dry mouth relating to appetite or thirst.Comparative measures of harm were known to be unlikely, and we therefore calculated the proportion of participants experiencing each of the adverse events of interest with each opioid, and for all four opioid drugs combined.
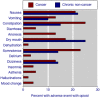
Main results: We included 77 studies with 5619 randomised participants. There was potential bias in most studies, with small size being the most common; individual treatment groups had fewer than 50 participants in 60 studies. Participants were relatively young, with mean age in the studies typically between 50 and 70 years. Multiple major problems with adverse event reporting were found, including failing to report adverse events in all participants who received medication, all adverse events experienced, how adverse events were collected, and not defining adverse event terminology or whether a reporting system was used.Direct measures of patient consciousness, patient appetite, or thirst were not apparent. For opioids used to treat cancer pain adverse event incidence rates were 25% for constipation, 23% for somnolence, 21% for nausea, 17% for dry mouth, and 13% for vomiting, anorexia, and dizziness. Asthenia, diarrhoea, insomnia, mood change, hallucinations and dehydration occurred at incidence rates of 5% and below.
Authors' conclusions: We found no direct evidence that opioids affected patient consciousness, appetite or thirst when used to treat cancer pain. However, somnolence, dry mouth, and anorexia were common adverse events in people with cancer pain treated with morphine, fentanyl, oxycodone, or codeine.We are aware that there is an important literature concerning the problems that exist with adverse event measurement, reporting, and attribution. Together with the known complications concerning concomitant medication, data collection and reporting, and nomenclature, this means that these adverse events cannot always be attributed unequivocally to the use of opioids, and so they provide only a broad picture of adverse events with opioids in cancer pain. The research agenda includes developing definitions for adverse events that have a spectrum of severity or importance, and the development of appropriate measurement tools for recording such events to aid clinical practice and clinical research.
Conflict of interest statement
SD, RAM, and PW have received research support from charities, government, and industry sources at various times. RAM, and PW have consulted for various pharmaceutical companies. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions.
Figures

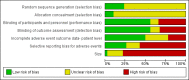

Update of
References
References to studies included in this review
Ahmedzai 1997 {published data only}
Ahmedzai 2012 {published data only}
-
- Ahmedzai SH, Nauck F, Bar‐Sela G, Bosse B, Leyendecker P, Hopp M. A randomized, double‐blind, active‐controlled, double‐dummy, parallel‐group study to determine the safety and efficacy of oxycodone/naloxone prolonged‐release tablets in patients with moderate/severe, chronic cancer pain. Palliative Medicine 2012;26:50‐60. [DOI: 10.1177/0269216311418869] - DOI - PMC - PubMed
Arkinstall 1989 {published data only}
-
- Goughnour BR, Arkinstall WW, Stewart JH. Analgesic response to single and multiple doses of controlled release morphine tablets and morphine oral solution in cancer patients. Cancer 1989;63(Suppl 11):2294‐7. - PubMed
Babul 1998 {published data only}
-
- Babul N, Provencher L, Laberge F, Harsanyi Z, Moulin D. Comparative efficacy and safety of controlled‐release morphine suppositories and tablets in cancer pain. Journal of Clinical Pharmacology 1998;38(1):74‐81. - PubMed
Boureau 1992 {published data only}
-
- Boureau F, Saudubray F, D'Arnoux C, Vedrenne J, Estève M, Roquefeuil B, et al. A comparative study of controlled‐release morphine (CRM) suspension and CRM tablets in chronic cancer pain. Journal of Pain and Symptom Management 1992;7(7):393‐9. - PubMed
Broomhead 1997 {published data only}
-
- Broomhead A, Kerr R, Tester W, O'Meara P, Maccarrone C, Bowles R, et al. Comparison of a once‐a‐day sustained‐release morphine formulation with standard oral morphine treatment for cancer pain. Journal of Pain and Symptom Management 1997;14(2):63‐73. - PubMed
Bruera 1998 {published data only}
-
- Bruera E, Belzile M, Pituskin E, Fainsinger R, Darke A, Harsanyi Z, et al. Randomized, double‐blind, cross‐over trial comparing safety and efficacy of oral controlled‐release oxycodone with controlled‐release morphine in patients with cancer pain. Journal of Clinical Oncology 1998;16(10):3222‐9. - PubMed
Bruera 2004 {published data only}
-
- Bruera E, Palmer JL, Bosnjak S, Rico MA, Moyano J, Sweeney C, et al. Methadone versus morphine as a first‐line strong opioid for cancer pain: a randomized, double‐blind study. Journal of Clinical Oncology 2004;22(1):185‐92. - PubMed
Carlson 1990 {published data only}
-
- Carlson RW, Borrison RA, Sher HB, Eisenberg PD, Mowry PA, Wolin EM. A multiinstitutional evaluation of the analgesic efficacy and safety of ketorolac tromethamine, acetaminophen plus codeine, and placebo in cancer pain. Pharmacotherapy 1990;10(3):211‐6. - PubMed
Cundiff 1989 {published data only}
-
- Cundiff D, McCarthy K, Savarese JJ, Kaiko R, Thomas G, Grandy R, et al. Evaluation of a cancer pain model for the testing of long‐acting analgesics. The effect of MS Contin in a double‐blind, randomized crossover design. Cancer 1989;63(11 Suppl):2355‐9. - PubMed
-
- Cundiff D, Savarese J, Grandy R, MacCarthy K, R. Kaiko R, G. Thomas G, et al. Evaluation of a controlled‐ and immediate release morphine and a unique well‐controlled study design in cancer pain. Journal of Pain and Symptom Management 1988;3((Suppl)):18.
Currow 2007 {published data only}
-
- Currow DC, Plummer JL, Cooney NJ, Gorman D, Glare PA. A randomized, double‐blind, multi‐site, crossover, placebo‐controlled equivalence study of morning versus evening once‐daily sustained‐release morphine sulfate in people with pain from advanced cancer. Journal of Pain and Symptom Management 2007;34:17‐23. - PubMed
Dale 2009 {published data only}
-
- Dale O, Piribauer M, Kaasa S, Moksnes K, Knobel H, Klepstad P. A double‐blind, randomized, crossover comparison between single‐dose and double‐dose immediate‐release oral morphine at bedtime in cancer patients. Journal of Pain and Symptom Management 2009;37:68‐76. - PubMed
De Conno 1995 {published data only}
-
- Conno F, Ripamonti C, Saita L, MacEachern T, Hanson J, Bruera E. Role of rectal route in treating cancer pain: a randomized crossover clinical trial of oral versus rectal morphine administration in opioid‐naive cancer patients with pain. Journal of Clinical Oncology 1995;13(4):1004‐8. - PubMed
Dellemijn 1994 {published data only}
-
- Dellemijn PL, Verbiest HB, Vliet JJ, Roos PJ, Vecht CJ. Medical therapy of malignant nerve pain. A randomised double‐blind explanatory trial with naproxen versus slow‐release morphine. European Journal of Cancer 1994;30A(9):1244‐50. - PubMed
Deschamps 1992 {published data only}
-
- Deschamps M, Band PR, Hislop TG, Rusthoven J, Iscoe N, Warr D. The evaluation of analgesic effects in cancer patients as exemplified by a double‐blind, crossover study of immediate‐release versus controlled‐release morphine. Journal of Pain and Symptom Management 1992;7(7):384‐92. - PubMed
Dhaliwal 1995 {published data only}
-
- Dhaliwal HS, Sloan P, Arkinstall WW, Thirlwell MP, Babul N, Harsanyi Z, et al. Randomized evaluation of controlled‐release codeine and placebo in chronic cancer pain. Journal of Pain and Symptom Management 1995;10(8):612‐23. - PubMed
Ferrell 1989 {published data only}
-
- Ferrell B, Wisdom C, Wenzl C, Brown J. Effects of controlled released morphine on quality of life for cancer pain. Oncology Nursing Forum 1989;16(4):521‐6. - PubMed
Finn 1993 {published data only}
-
- Finn JW, Walsh TD, MacDonald N, Bruera E, Krebs LU, Shepard KV. Placebo‐blinded study of morphine sulfate sustained‐release tablets and immediate‐release morphine sulfate solution in outpatients with chronic pain due to advanced cancer. Journal of Clinical Oncology 1993;11(5):967‐72. - PubMed
Flöter 1997 {published data only}
-
- Flöter T, Koch EMW, and the Kap‐Cas study group. Comparison of two oral morphine formulations for chronic severe pain of malignant and non malignant origin Kapanol (R) vs MST (R). Clinical Drug Investigation 1997;14(3):183‐91.
Gabrail 2004 {published data only}
-
- Gabrail NY, Dvergsten C, Ma T, Frailey A, Ahdieh H. Oxymorphone extended‐release (ER) provides safe, effective, and rapid analgesia during opioid radiation: results of a randomized, double‐blind, crossover, comparative study with oxycodone controlled‐release (CR) [abstract]. 2003; Vol. 22:737.
-
- Gabrail Nashat Y, Dvergsten Chris, Ahdieh Harry. Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Current Medical Research & Opinion 2004;20(6):911‐8. - PubMed
Gillette 1997 {published data only}
-
- Gillette JF, Ferme C, Moisy N, Mignot L, Schach R, Vignaux J, et al. Double‐blind crossover clinical and pharmacokinetic comparison of oral morphine syrup and sustained release morphine sulfate capsules in patients with cancer‐related pain. Clinical Drug Investigation 1997;14(Suppl 1):22‐7.
Gourlay 1997 {published data only}
-
- Gourlay GK, Cherry DA, Onley MM, Tordoff SG, Conn DA, Hood GM, et al. Pharmacokinetics and pharmacodynamics of twenty‐four‐hourly Kapanol compared to twelve‐hourly MS Contin in the treatment of severe cancer pain. Pain 1997;69(3):295‐302. - PubMed
Guo‐Zhu 1997 {published data only}
-
- Guo‐Zhu Xu, Zhi‐Ji Cai, Yan‐Ping Deng, Jun Hou, Guang‐Ru Xie, Shu‐Jun Liu. Clinical Evaluation of the analgesic effect of sustained release morphine sulfate microgranules in patients with terminal cancer. Clinical Drug Investigation 1997;Suppl: 14(1):34‐42.
Hagen 1997 {published data only}
-
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled‐release oxycodone formulation and controlled‐release hydromorphone in the treatment of cancer pain. Cancer 1997;79(7):1428‐37. - PubMed
Hagen 2005 {published data only}
-
- Hagen NA, Thirlwell M, Eisenhoffer J, Quiqley P, Harsanyi Z, Darke A. Efficacy, safety, and steady state pharmacokinetics of once‐a ‐day controlled release morphine (MS Contin XL) in cancer pain. Journal of Pain and Symptom Management 2005;29(1):80‐90. - PubMed
Hanks 1987 {published data only}
-
- Hanks GW, Twycross RG, Bliss JM. Controlled release morphine tablets: a double blind trial in patients with advanced cancer. Anaesthesia 1987;42(8):840‐4. - PubMed
Hanks 1995 {published data only}
-
- Hanks GW, Hanna M, Finlay I, Radstone DJ, Keeble T. Efficacy and pharmacokinetics of a new controlled‐release morphine sulfate 200‐mg tablet. Journal of Pain and Symptom Management 1995;10(1):6‐12. - PubMed
Hanna 2008 {published data only}
Harris 2003 {published data only}
-
- Harris JT, Suresh Kumar K, Rajagopal MR. Intravenous morphine for rapid control of severe cancer pain. Palliative Medicine 2003;17:248‐56. - PubMed
Heiskanen 1997 {published data only}
Homsi 2010 {published data only}
-
- Homsi J, Walsh D, Lasheen W, Nelson K A, Rybicki L A, Bast J, et al. A comparative study of 2 sustained‐release morphine preparations for pain in advanced cancer. American Journal of Hospice and Palliative Care 2010;27:99‐105. - PubMed
Hoskin 1989 {published data only}
-
- Hoskin PJ, Poulain P, Hanks GW. Controlled release morphine in cancer pain. Is a loading dose required when the formulation is changed?. Anaesthesia 1989;44(11):897‐901. - PubMed
Imanaka 2013 {published data only}
-
- Imanaka K, Tominaga Y, Etropolski M, Hove I, Ohsaka M, Wanibe M. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor‐related pain. Current Medical Research and Opinion 2013;29(10):1399‐409. [DOI: 10.1185/03007995.2013.831816] - DOI - PubMed
Kalso 1990 {published data only}
-
- Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clinical Pharmacology and Therapeutics 1990;47(5):639‐46. - PubMed
Kaplan 1998 {published data only}
-
- Kaplan R, Parris WC, Citron ML, Zhukovsky D, Reder RF, Buckley BJ, et al. Comparison of controlled‐release and immediate‐release oxycodone tablets in patients with cancer pain. Journal of Clinical Oncology 1998;16(10):3230‐7. - PubMed
Kerr 2000 {published data only}
-
- Kerr RO, Tester WJ. A patient preference study comparing two extended‐release morphine sulfate formulations (once‐daily Kadian™ versus twice‐daily MS Contin™ for cancer pain. Clinical Drug Investigation 2000;19(1):25‐32.
Klepstad 2003 {published data only}
-
- Klepstad P, Kaasa S, Jystad A, Hval B, Borchgrevink PC. Immediate‐ or sustained‐release morphine for dose finding during start of morphine to cancer patients: a randomized, double‐blind trial. Pain 2003;101(1‐2):193‐8. - PubMed
Knudsen 1985 {published data only}
-
- Henriksen H, Knudsen J. Controlled evaluation and ongoing experience with sustained release morphine in patients with advanced cancer pain. Advances in Cancer Pain Management. The International Symposium on Pain Control. Toronto: Purdue Frederick Inc, 1986.
-
- Knudsen J, Mortensen SM, Eikard B, Henriksen H. Slow‐release morphine tablets compared with conventional morphine tablets in the treatment of cancer pain [Morfin‐depottabletter og konventionelle morfintabletter ved cancersmerter]. Ugeskr Læger 1985;147(9):780‐4. - PubMed
Kongsgaard 1998 {published data only}
-
- Kongsgaard UE, Poulain P. Transdermal fentanyl for pain control in adults with chronic cancer pain. European Journal of Pain 1998;2(1):53‐62. - PubMed
Kossman 1983 {published data only}
-
- Kossman B, Dick W, Bowdler I, Kilian J, Hecht M. Modern aspects of morphine therapy. Advances in Morphine Therapy. The 1983 International Symposium on Pain Control. Royal Society of Medicine International Congress and Symposium Series no 64. London: Royal Society of Medicine, 1983:73‐81.
Kress 2008 {published data only}
-
- Kress HG, Laage D, Hoerauf KH, Nolte T, Heiskanen T, Petersen R, et al. A randomized, open, parallel group, multicenter trial to investigate analgesic efficacy and safety of a new transdermal fentanyl patch compared to standard opioid treatment in cancer pain. Journal of Pain and Symptom Management 2008;36(3):268‐79. [DOI: 10.1016/j.jpainsymman.2007.10.023] - DOI - PubMed
Lauretti 2003 {published data only}
Leppart 2001 {published data only}
-
- Leppart W. Analgesic efficacy and side effects of oral tramadol and morphine administered orally in the treatment of cancer pain. Nowotwory 2001;51(3):257‐66.
Melzack 1979 {published data only}
Mercadante 1998 {published data only}
-
- Mercadante S, Salvaggio L, Dardanoni G, Agnello A, Garofalo S. Dextropropoxyphene versus morphine in opioid‐naive cancer patients with pain. Journal of Pain and Symptom Management 1998;15(2):76‐81. - PubMed
Mercadante 2008 {published data only}
Mercadante 2010 {published data only}
-
- Mercadante S, Tirelli W, David F, Arcara C, Fulfaro F, Casuccio A, et al. Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clinical Journal of Pain 2010;26(9):294‐7. - PubMed
Mignault 1995 {published data only}
-
- Mignault GG, Latreille J, Viguie F, Richer P, Lemire F, Harsanyi Z, et al. Control of cancer‐related pain with MS Contin: a comparison between 12‐hourly and 8‐hourly administration. Journal of Pain and Symptom Management 1995;10(6):416‐22. - PubMed
Mizuguchi 1990 {published data only}
-
- Mizuguchi K, Takeda F, Hiraga K, Nakashima M. Utility evaluation of morphine hydrochloride suppository, AN‐631, in the treatment of cancer pain. Rinshou Igaku 1990;6(11):2357‐76.
Moriarty 1999 {published data only}
-
- Moriarty M, McDonald CJ, Miller AJ. A randomised crossover comparison of controlled release hydromorphone tablets with controlled release morphine tablets in patients with cancer pain. Journal of Clinical Research 1999;2:1‐8.
Mucci LoRusso 1998 {published data only}
-
- Mucci LoRusso P, Berman BS, Silberstein PT, Citron ML, Bressler L, Weinstein SM, et al. Controlled‐release oxycodone compared with controlled‐release morphine in the treatment of cancer pain: A randomized, double‐blind, parallel‐group study. European Journal of Pain 1998;2:239‐49. - PubMed
Mystakidou 2005 {published data only}
-
- Mystakidou K, Katsouda E, Kouloulias V, Kouvaris J, Tsiatas M, Vlahos L. Comparison of transdermal fentanyl with codeine/paracetamol, in combination with radiotherapy, for the management of metastatic bone pain. Journal of Opioid Management 2005;1(4):204‐10. - PubMed
O'Brien 1997 {published data only}
-
- O'Brien T, Mortimer PG, McDonald CJ, Miller AJ. A randomized crossover study comparing the efficacy and tolerability of a novel once‐daily morphine preparation (MXL capsules) with MST Continus tablets in cancer patients with severe pain. Palliative Medicine 1997;11(6):475‐82. - PubMed
Oztürk 2008 {published data only}
-
- Oztürk T, Karadibak K, Catal D, Cakan A, Tugsavul F, Cirak K. Comparison of TD‐fentanyl with sustained‐release morphine in the pain treatment of patients with lung cancer. Agri 2008;20(3):20‐5. - PubMed
Panich 1993 {published data only}
-
- Panich A, Charnvej L. Comparison of morphine slow release tablet (MST) and morphine sulphate solution (MSS) in the treatment of cancer pain. Journal of Medical Association of Thailand 1993;76(12):672‐6. - PubMed
Parris 1998 {published data only}
-
- Parris WC, Johnson BW Jr, Croghan MK, Moore MR, Khojasteh A, Reder RF, et al. The use of controlled‐release oxycodone for the treatment of chronic cancer pain: a randomized, double‐blind study. Journal of Pain & Symptom Management 1998;16(4):205‐11. - PubMed
Pistevou‐Gompaki 2004 {published data only}
-
- Pistevou‐Gompaki K, Kouloulias VE, Varveris C, Mystakidou K, Georgakopoulos G, Eleftheriadis N, et al. Radiotherapy plus either transdermal fentanyl or paracetamol and codeine for painful bone metastases: a randomised study of pain relief and quality of life. Current Medical Research and Opinion 2004;20(2):159‐63. [DOI: 10.1185/030079903125002829] - DOI - PubMed
Portenoy 1989 {published data only}
-
- Portenoy R, Maldonado M, Fitzmartin R, Kaiko R, Kanner R. Oral controlled release morphine sulfate. Analgesic efficacy and side effects of a 100 mg tablet in cancer pain patients. Cancer 1989;63(Suppl: 11):2284‐8. - PubMed
-
- Portenoy RK, Maldonado M, Fitzmartin R, Kaiko R, Kanner R. Controlled‐release morphine sulfate: analgesic efficacy and side effects of a 100 mg tablet. Journal of Pain and Symptom Management 1988;3(3):16 (supplement). - PubMed
Rico 2000 {published data only}
-
- Rico MA, Cura MA, Harbst H, Palominos A, Figueroa M, Kramer V. Assessment of tramadol as an alternative opioid instead of codeine at the second step of the WHO analgesic scale [Evaluación de tramadol como un opioide alternativoa la codeína en el segundo peldaño de la escalera analgésica de la OMS]. Revista de la Sociedad Espanola del Dolor 2000;7:345‐53.
Ridgway 2010 {published data only}
-
- Ridgway D, Sopata M, Burneckis A, Jespersen L, Andersen C. Clinical efficacy and safety of once‐daily dosing of a novel, prolonged‐release oral morphine tablet compared with twice‐daily dosing of a standard controlled‐release morphine tablet in patients with cancer pain: a randomized, double‐blind, exploratory crossover study. Journal of Pain and Symptom Management 2010;39:712‐20. - PubMed
Rodriguez 1994 {published data only}
-
- Rodriguez M, Barutell C, Rull M, Gálvez R, Pállares J, Vidal F, et al. Efficacy and tolerance of oral dipyrone versus oral morphine for cancer pain. European Journal of Cancer 1994;30A(5):584‐7. - PubMed
Rodriguez 2007 {published data only}
-
- Rodriguez RF, Bravo LE, Castro F, Montoya O, Castillo JM, Castillo MP, Daza P, Restrepo JM, Rodriguez MF. Incidence of weak opioids adverse events in the management of cancer pain: a double‐blind comparative trial. Journal of Palliative Medicine 2007;10(1):56‐60. - PubMed
-
- Rodriguez RF, Castillo JM, Castillo MP, Montoya O, Daza P, Rodríguez MF, et al. Hydrocodone/acetaminophen and tramadol chlorhydrate combination tablets for the management of chronic cancer pain: a double‐blind comparative trial. Clinical Journal of Pain 2008;24(1):1‐4. [DOI: 10.1097/AJP.0b013e318156ca4d] - DOI - PubMed
-
- Rodriguez RF, Castillo JM, Pilar Castillo M, Nuñez PD, Rodriguez MF, Restrepo JM, et al. Codeine/acetaminophen and hydrocodone/acetaminophen combination tablets for the management of chronic cancer pain in adults: a 23‐day, prospective, double‐blind, randomized, parallel‐group study. Clinical Therapeutics 2007;29(4):581‐7. - PubMed
Salzman 1999 {published data only}
-
- Salzman RT, Roberts MS, Wild J, Fabian C, Reder RF, Goldenheim PD. Can a controlled‐release oral dose form of oxycodone be used as readily as an immediate‐release form for the purpose of titrating to stable pain control?. Journal of Pain & Symptom Management 1999;18(4):271‐9. - PubMed
Smith 1991 {published data only}
-
- Smith KJ, Miller AJ, McKellar J, Court M. Morphine at gramme doses: kinetics, dynamics and clinical need. Postgraduate Medical Journal 1991;67(Suppl 2):S55‐9. - PubMed
Stambaugh 2001 {published data only}
-
- Stambaugh JE, Reder RF, Stambaugh M. Double‐blind, randomized, two‐period crossover efficacy and pharmacokinetic comparison of immediate‐release oxycodone (IR) and controlled‐release oxycodone (CR) in cancer patients with pain. Clinical Pharmacology & Therapeutics 1997;61(2):197.
-
- Stambaugh JE, Reder RF, Stambaugh MD, Stambaugh H, Davis M. Double‐blind, randomized comparison of the analgesic and pharmacokinetic profiles of controlled‐ and immediate‐release oral oxycodone in cancer pain patients. Journal of Clinical Pharmacology 2001;41(5):500‐6. - PubMed
Thirlwell 1989 {published data only}
-
- Thirlwell MP, Sloan PA, Maroun JA, Boos GJ, Besner JG, Stewart JH, et al. Pharmacokinetics and clinical efficacy of oral morphine solution and controlled‐release morphine tablets in cancer patients. Cancer 1989;63(Suppl 11):2275‐83. - PubMed
Todd 2002 {published data only}
-
- Todd J, Rees E, Gwilliam B, Davies A. An assessment of the efficacy and tolerability of a 'double dose' of normal‐release morphine sulphate at bedtime.. Palliative Medicine 2002;16:507‐12. - PubMed
Vainio 1988 {published data only}
-
- Vainio A, Tigerstedt I. Opioid treatment for radiating cancer pain: oral administration vs. epidural techniques. Acta Anaesthesiologica Scandinavica 1988;32(3):179‐85. - PubMed
van Seventer 2003 {published data only}
Ventafridda 1986 {published data only}
-
- Ventafridda V, Ripamonti C, Bianchi M, Sbanotto A, Conno F. A randomized study on oral administration of morphine and methadone in the treatment of cancer pain. Journal of Pain and Symptom Management 1986;1(4):203‐7. - PubMed
Ventafridda 1989 {published data only}
-
- Ventafridda V, Saita L, Barletta L, Sbanotto A, Conno F. Clinical observations on controlled release morphine in cancer pain. Journal of Pain and Symptom Management 1989;4(3):124‐9. - PubMed
Vielvoye‐Kerkmeer 2002 {published data only}
-
- Vielvoye‐Kerkmeer A, Tinteren H, Mattern C, Schüller J, Farnell A. Sustained release morphine in cancer pain. European Journal of Palliative Care 2002;9(4):137‐40.
Walsh 1985 {published data only}
-
- Walsh TD. A controlled study of MST Continus tablets for chronic pain in advanced cancer. In: Wilkes E editor(s). Advances in Morphine Therapy: International Symposium on Pain Control. London: Royal Society of Medicine, 1984:99‐102.
-
- Walsh TD. Clinical evaluation of slow release morphine tablets. Advances in Pain Research and Therapy 1985;9:727‐31.
Walsh 1992 {published data only}
-
- Walsh TD, MacDonald N, Bruera E, Shepard KV, Michaud M, Zanes R. A controlled study of sustained‐release morphine sulfate tablets in chronic pain from advanced cancer. American Journal of Clinical Oncology 1992;15(3):268‐72. - PubMed
Wilder‐Smith1994 {published data only}
-
- Wilder‐Smith CH, Schimke J, Osterwalder B, Senn HJ. Oral tramadol, a mu‐opioid agonist and monoamine reuptake‐blocker, and morphine for strong cancer‐related pain. Annals of Oncology 1994;5(2):141‐6. - PubMed
Wilkinson 1992 {published data only}
-
- Wilkinson TJ, Robinson BA, Begg EJ, Duffull SB, Ravenscroft PJ, Schneider JJ. Pharmacokinetics and efficacy of rectal versus oral sustained‐release morphine in cancer patients. Cancer Chemotherapy and Pharmacology 1992;31(3):251‐4. - PubMed
Wong 1997 {published data only}
-
- Wong JO, Chiu GL, Tsao CJ, Chang CL. Comparison of oral controlled‐release morphine with transdermal fentanyl in terminal cancer pain. Acta Anaesthesiologica Sinica 1997;35(3):25‐32. - PubMed
References to studies excluded from this review
Beaver 1978 I {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):92‐100. - PubMed
Beaver 1978 II {published data only}
-
- Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. Journal of Pharmacology and Experimental Therapeutics 1978;207(1):101‐8. - PubMed
Capretti 1970 {published data only}
-
- Capretti G, Frigerio G. Controlled clinical study of the analgesic activity of a new drug (Z. 424) in pain caused by neoplasms [Studio clinico controllato dell'attivita analgesica di un nuovo farmaco (Z. 424) nel dolore da neoplasie]. La Clinica Terapeutica 1970;52(4):361‐9. - PubMed
Chen 2003 {published data only}
-
- Chen Y, Zhu W, Liang H, Wu G. The analgesic effect of ibuprofen‐codeine sustained release tablets on postoperative and cancer pain. Chinese Journal of Clinical Rehabilitation 2003;7(8):1290‐1.
Coluzzi 2001 {published data only}
Jochimsen 1978 I {published data only}
-
- Jochimsen PR, Lawton RL, VerSteeg K, Noyes R Jr. Effect of benzopyranoperidine, a delta‐9‐THC congener, on pain. Clinical Pharmacology and Therapeutics 1978;24(2):223‐7. - PubMed
Leow 1995 {published data only}
-
- Leow KP, Cramond T, Smith MT. Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anesthesia & Analgesia 1995;80(2):296‐302. - PubMed
Moertel 1971 {published data only}
-
- Moertel CG, Ahmann DL, Taylor WF, Schwartau N. Aspirin and pancreatic cancer pain. Gastroenterology 1971;60(4):552‐3. - PubMed
Noyes 1975 {published data only}
Stambaugh 1987 {published data only}
-
- Stambaugh JE Jr, McAdams J. Comparison of the analgesic efficacy and safety oral ciramadol, codeine, and placebo in patients with chronic cancer pain. Journal of Clinical Pharmacology 1987;27(2):162‐6. - PubMed
Staquet 1971 {published data only}
-
- Staquet M, Luyckx A, Cauwenberge H. A double‐blind comparison of alclofenac, pentazocine, and codeine with placebo control in pathologic pain. Journal of clinical pharmacology and new drugs 1971;11(6):450‐5. - PubMed
Staquet 1978 {published data only}
-
- Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical Pharmacology and Therapeutics 1978;23(4):397‐401. - PubMed
Staquet 1993 {published data only}
-
- Staquet M, Renaud A. Double‐blind randomized trial of piroxicam and codeine in cancer pain. Current Theraputics and Research 1993;53(4):435‐40.
Additional references
AUREF 2012
-
- PaPaS author and referee guidance. http://papas.cochrane.org/papas‐documents (accessed 14 March 2014).
Avorn 2008
Bailey 2005
-
- Bailey FA, Burgio KL, Woodby LL, Williams BR, Redden DT, Kovac SH, et al. Improving processes of hospital care during the last hours of life. Arch Intern Med 2005;165:1722‐27. - PubMed
Bookbinder 2005
-
- Bookbinder M, Blank A, Arney E, Wollner D, Lesage P, McHugh M, et al. Improving end of life care: development and pilot test of a clinical pathway. J Pain Symptom Manage 2005;29:529‐43. - PubMed
Chan 2013
Cook 1995
Costantini 2014
Derry 2008
-
- Derry S. Adverse events. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:67‐84. [ISBN: 978‐0‐931092‐69‐5]
DH 2013
-
- Anon. More care, less pathway. A review of the Liverpool Care Pathway. Report by Baroness Julia Neuberger (Chair) 2013.
Edwards 1999
Ellershaw 2003
George 2014
Hadley 2012
Higgins 2011
-
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2001
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Knights 2013
Loke 2001
McQuay 1997
Meyer 1996
-
- Meyer FP, Tröger U, Röhl FW. Adverse nondrug reactions: an update. Clinical Pharmacology and Therapeutics 1996;60(3):347‐52. - PubMed
Moore 1998
-
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2005
Moore 2009
-
- Moore A, Bjarnason I, Cryer B, Garcia‐Rodriguez L, Goldkind L, Lanas A, et al. Evidence for endoscopic ulcers as meaningful surrogate endpoint for clinically significant upper gastrointestinal harm. Clinical Gastroenterology and Hepatology 2009;7(11):1156‐63. [DOI: 10.1016/j.cgh.2009.03.032.] - DOI - PubMed
Moore 2010
-
- Andrew Moore R, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, McQuay H, ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] - DOI - PubMed
Moore 2013
Murray 2008
Nuovo 2007
Nüesch 2010
Olsen 1999
-
- Olsen H, Klemetsrud T, Stokke HP, Tretli S, Westheim A. Adverse drug reactions in current antihypertensive therapy: a general practice survey of 2586 patients in Norway. Blood Pressure 1999;8(2):94‐101. - PubMed
Portenoy 1999
-
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999;353:1695‐1700. - PubMed
Regnard 2014
Reidenberg 1968
-
- Reidenberg MM, Lowenthal DT. Adverse nondrug reactions. New England Journal of Medicine 1968;279(13):678‐9. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rief 2006
Rief 2009
-
- Rief W, Nestoriuc Y, Lilienfeld‐Toal A, Dogan I, Schreiber F, Hofmann SG. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta‐analysis. Drug Safety 2009;32(11):1041‐56. [DOI: 10.2165/11316580-000000000-00000] - DOI - PubMed
Schmidt‐Hansen 2010
-
- Schmidt‐Hansen M, Bennett MI, Arnold S. Oxycodone for cancer‐related pain. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD003870.pub3] - DOI
Schremmer 2013
Tassi 2001
-
- Tassi P, Muzet A. Defining the states of consciousness. Neuroscience and Biobehavioral Reviews 2001;25(2):175‐91. - PubMed
Tramèr 1997
Tramèr 2000
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

