Molecular targets of aspirin and cancer prevention
- PMID: 24874482
- PMCID: PMC4090734
- DOI: 10.1038/bjc.2014.271
Molecular targets of aspirin and cancer prevention
Abstract
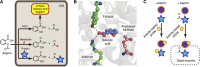
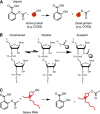
Salicylates from plant sources have been used for centuries by different cultures to treat a variety of ailments such as inflammation, fever and pain. A chemical derivative of salicylic acid, aspirin, was synthesised and mass produced by the end of the 19th century and is one of the most widely used drugs in the world. Its cardioprotective properties are well established; however, recent evidence shows that it can also act as a chemopreventive agent. Its antithrombotic and anti-inflammatory actions occur through the inhibition of cyclooxygenases. The precise mechanisms leading to its anticancer effects are not clearly established, although multiple mechanisms affecting enzyme activity, transcription factors, cellular signalling and mitochondrial functions have been proposed. This review presents a brief account of the major COX-dependent and independent pathways described in connection with aspirin's anticancer effects. Aspirin's unique ability to acetylate biomolecules besides COX has not been thoroughly investigated nor have all the targets of its primary metabolite, salicylic acid been identified. Recent reports on the ability of aspirin to acetylate multiple cellular proteins warrant a comprehensive study to investigate the role of this posttranslational modification in its anticancer effects. In this review, we also raise the intriguing possibility that aspirin may interact and acetylate cellular molecules such as RNA, and metabolites such as CoA, leading to a change in their function. Research in this area will provide a greater understanding of the mechanisms of action of this drug.
Figures

References
-
- Abdelrahim M, Safe S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol Pharmacol. 2005;68 (2):317–329. - PubMed
-
- Alfonso LF, Srivenugopal KS, Arumugam TV, Abbruscato TJ, Weidanz JA, Bhat GJ. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. Int J Oncol. 2009;34 (3):597–608. - PubMed
-
- Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins?(Review) Mol Med Rep. 2009;2 (4):533–537. - PubMed
-
- Archer SY, Hodin RA. Histone acetylation and cancer. Curr Opin Genet Dev. 1999;9 (2):171–174. - PubMed
-
- Arnold JT, Wilkinson BP, Sharma S, Steele VE. Evaluation of chemopreventive agents in different mechanistic classes using a rat tracheal epithelial cell culture transformation assay. Cancer Res. 1995;55 (3):537–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

