Concordance between RT-PCR-based detection of respiratory viruses from nasal swabs collected for viral testing and nasopharyngeal swabs collected for bacterial testing
- PMID: 24875136
- PMCID: PMC4055503
- DOI: 10.1016/j.jcv.2014.04.011
Concordance between RT-PCR-based detection of respiratory viruses from nasal swabs collected for viral testing and nasopharyngeal swabs collected for bacterial testing
Abstract
Background: Epidemiologic studies of respiratory infections frequently rely on separate sample collections for the detection of bacteria and viruses. The requirement for two specimens presents cost, logistical, and acceptability challenges.
Objectives: To determine the agreement in detection of respiratory viruses using RT-PCR between two different types of samples collected on the same day: nasal swabs preserved in viral transport medium (NS) and nasopharyngeal swabs preserved in skim milk-tryptone-glucose-glycerol [STGG] medium (NP), the current standard for pneumococcal colonization studies.
Study design: Paired NS and NP samples were collected between May 2009 and September 2011 as part of the RESPIRA-PERU study, a large prospective cohort of Andean children <3 years of age. NS samples used polyester swabs and viral transport medium whereas NP samples used rayon wire-handled swabs and STGG medium. Samples were tested for influenza, human metapneumovirus (MPV), respiratory syncytial virus (RSV), human rhinovirus (HRV), parainfluenza virus 3 (PIV3) and adenovirus (ADV) using real-time RT-PCR. We calculated the agreement, and compared cycle thresholds (CT) between NP and NS samples.
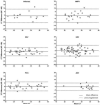
Results: Among 226 paired NP-NS samples, we observed very high agreement with a Kappa statistic ranging from 0.71 for ADV to 0.97 for MPV. CT values were similar for both strategies.
Conclusions: NP samples preserved in STGG provide a simple and reliable strategy for identification of both pneumococcus and respiratory viruses. This single specimen collection strategy could be used for epidemiologic studies, especially in resource-limited settings. Furthermore, archived NP-STGG specimens from previous studies could be reliably tested by RT-PCR for viruses.
Keywords: Children; Epidemiology; Nasal swab; Nasopharyngeal swab; Respiratory virus.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
References
-
- O’Brien KL, Nohynek H. Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:133–40. - PubMed
-
- Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32:165–79. - PubMed
-
- Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. PediatrInfectDisJ. 2004;23:S188–S92. - PubMed
-
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. The New England journal of medicine. 2006;355:31–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

