Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model
- PMID: 24875186
- PMCID: PMC4038468
- DOI: 10.1371/journal.pntd.0002897
Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model
Abstract
Background: The resident skin microbiota plays an important role in restricting pathogenic bacteria, thereby protecting the host. Scabies mites (Sarcoptes scabiei) are thought to promote bacterial infections by breaching the skin barrier and excreting molecules that inhibit host innate immune responses. Epidemiological studies in humans confirm increased incidence of impetigo, generally caused by Staphylococcus aureus and Streptococcus pyogenes, secondary to the epidermal infestation with the parasitic mite. It is therefore possible that mite infestation could alter the healthy skin microbiota making way for the opportunistic pathogens. A longitudinal study to test this hypothesis in humans is near impossible due to ethical reasons. In a porcine model we generated scabies infestations closely resembling the disease manifestation in humans and investigated the scabies associated changes in the skin microbiota over the course of a mite infestation.
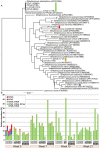
Methodology/principal findings: In a 21 week trial, skin scrapings were collected from pigs infected with S. scabies var. suis and scabies-free control animals. A total of 96 skin scrapings were collected before, during infection and after acaricide treatment, and analyzed by bacterial 16S rDNA tag-encoded FLX-titanium amplicon pyrosequencing. We found significant changes in the epidermal microbiota, in particular a dramatic increase in Staphylococcus correlating with the onset of mite infestation in animals challenged with scabies mites. This increase persisted beyond treatment from mite infection and healing of skin. Furthermore, the staphylococci population shifted from the commensal S. hominis on the healthy skin prior to scabies mite challenge to S. chromogenes, which is increasingly recognized as being pathogenic, coinciding with scabies infection in pigs. In contrast, all animals in the scabies-free cohort remained relatively free of Staphylococcus throughout the trial.
Conclusions/significance: This is the first experimental in vivo evidence supporting previous assumptions that establishment of pathogens follow scabies infection. Our findings provide an explanation for a biologically important aspect of the disease pathogenesis. The methods developed from this pig trial will serve as a guide to analyze human clinical samples. Studies building on this will offer implications for development of novel intervention strategies against the mites and the secondary infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Engelman D, Kiang K, Chosidow O, McCarthy J, Fuller C, et al. (2013) Toward the Global Control of Human Scabies: Introducing the International Alliance for the Control of Scabies. PLoS Negl Trop Dis 7(8): e2167 doi:10.1371/journal.pntd.0002167 - DOI - PMC - PubMed
-
- Hay RJ, Steer AC, Engelman D, Walton S (2012) Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect 18: 313–323. - PubMed
-
- Fuller LC (2013) Epidemiology of scabies. Curr Opin Infect Dis 26: 123–126. - PubMed
-
- Hicks MI, Elston DM (2009) Scabies. Dermatol Ther 22: 279–292. - PubMed
-
- Makigami K, Ohtaki N, Ishii N, Yasumura S (2009) Risk factors of scabies in psychiatric and long-term care hospitals: a nationwide mail-in survey in Japan. J Dermatol 36: 491–498. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

