Collecting duct principal cell transport processes and their regulation
- PMID: 24875192
- PMCID: PMC4284417
- DOI: 10.2215/CJN.05760513
Collecting duct principal cell transport processes and their regulation
Abstract
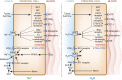
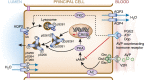
The principal cell of the kidney collecting duct is one of the most highly regulated epithelial cell types in vertebrates. The effects of hormonal, autocrine, and paracrine factors to regulate principal cell transport processes are central to the maintenance of fluid and electrolyte balance in the face of wide variations in food and water intake. In marked contrast with the epithelial cells lining the proximal tubule, the collecting duct is electrically tight, and ion and osmotic gradients can be very high. The central role of principal cells in salt and water transport is reflected by their defining transporters-the epithelial Na(+) channel (ENaC), the renal outer medullary K(+) channel, and the aquaporin 2 (AQP2) water channel. The coordinated regulation of ENaC by aldosterone, and AQP2 by arginine vasopressin (AVP) in principal cells is essential for the control of plasma Na(+) and K(+) concentrations, extracellular fluid volume, and BP. In addition to these essential hormones, additional neuronal, physical, and chemical factors influence Na(+), K(+), and water homeostasis. Notably, a variety of secreted paracrine and autocrine agents such as bradykinin, ATP, endothelin, nitric oxide, and prostaglandin E2 counterbalance and limit the natriferic effects of aldosterone and the water-retaining effects of AVP. Considerable recent progress has improved our understanding of the transporters, receptors, second messengers, and signaling events that mediate principal cell responses to changing environments in health and disease. This review primarily addresses the structure and function of the key transporters and the complex interplay of regulatory factors that modulate principal cell ion and water transport.
Keywords: collecting duct; epithelial cell; principal cell; renal physiology.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Palmer LG, Frindt G: Na+ and K+ transport by the renal connecting tubule. Curr Opin Nephrol Hypertens 16: 477–483, 2007 - PubMed
-
- Schuster VL, Stokes JB: Chloride transport by the cortical and outer medullary collecting duct. Am J Physiol 253: F203–F212, 1987 - PubMed
-
- Palmer LG, Patel A, Frindt G: Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol 16: 35–43, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

