The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation
- PMID: 24875228
- PMCID: PMC4135249
- DOI: 10.1038/eye.2014.110
The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation
Abstract
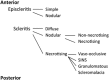
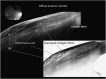
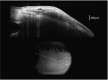
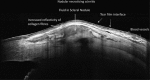
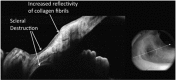
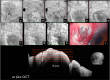
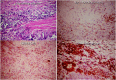
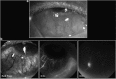
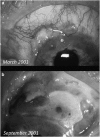
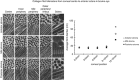
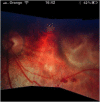
Recent advances in the understanding of the initiation and perpetuation of the immune response strongly suggest that all forms of noninfective immunologically induced scleral inflammation have a common origin. Analysis of the progress of patients with scleritis corroborates the current clinical classification that, together with studies of the immunohistology fluoresceine/ICG angiography, 3D proteoglycan, and keratan sulphate electron microscopy of scleritis, strongly suggests that from the initiation of the inflammatory process, necrotizing scleritis and diffuse and nodular scleritis not only pursue a different course but also have a different pathogenesis; nonnecrotizing scleritis being the consequence of an auto immune response, whereas necrotizing scleritis being the complication of an already present (if not always manifest), systemic immune-mediated systemic disease and its associated vasculitis. The increasing imaging capacity of anterior segment ocular coherence tomography (OCT) and en face OCT enables the changes occurring in the sclera during the course of the disease to be observed for the first time. These observations suggest that the inflammatory changes involve the potential suprachoroidal space between choroid and sclera, an observation supported by the presence of subscleral granulomas on histopathology. New imaging techniques have also been able to explain the changes seen in the cornea as a complication of scleritis. These findings have implications for investigation and the treatment of these conditions.
Figures








References
-
- Wiiliamson J. Incidence of eye disease in cases of connective tissue disease. Trans Ophthal Soc UK. 1974;94:742–752. - PubMed
-
- Watson PG, Hazleman BL, McKluskey P, Pavesio CE.The Sclera and Systemic Disorders3rd edn.JP Medical: London; 2012
-
- Sainz de la Maza M, Tauber J, Foster CS.The Sclera2nd ednSpringer Verlag: New York; 2012
-
- Wieringa WG, Wieringa JE, ten Dam-van Loon NH, Los LI. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology. 2013;120 (2:379–386. - PubMed
-
- Watson PG, Young RD. Scleral structure, organisation and disease. Exp Eye Res. 2004;78 (3:609–623. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

