Syntheses and radiosyntheses of two carbon-11 labeled potent and selective radioligands for imaging vesicular acetylcholine transporter
- PMID: 24875230
- PMCID: PMC4414325
- DOI: 10.1007/s11307-014-0748-x
Syntheses and radiosyntheses of two carbon-11 labeled potent and selective radioligands for imaging vesicular acetylcholine transporter
Abstract
Purpose: The vesicular acetylcholine transporter (VAChT) is a specific biomarker for imaging presynaptic cholinergic neurons. The syntheses and C-11 labeling of two potent enantiopure VAChT inhibitors are reported here.
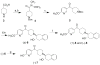
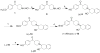
Procedures: Two VAChT inhibitors, (±)-2 and (±)-6, were successfully synthesized. A chiral HPLC column was used to resolve the enantiomers from each corresponding racemic mixture for in vitro characterization. The radiosyntheses of (-)-[(11)C]2 and (-)-[(11)C]6 from the corresponding desmethyl phenol precursor was accomplished using [(11)C]methyl iodide or [(11)C]methyl triflate, respectively.
Results: The synthesis of (-)-[(11)C]2 was accomplished with 40-50 % radiochemical yield (decay-corrected), SA > 480 GBq/μmol (EOB), and radiochemical purity >99 %. Synthesis of (-)-[(11)C]6 was accomplished with 5-10 % yield, SA > 140 GBq/μmol (EOB), and radiochemical purity >97 %. The radiosynthesis and dose formulation of each tracer was completed in 55-60 min.
Conclusions: Two potent enantiopure VAChT ligands were synthesized and (11)C-labeled with good radiochemical yield and specific activity.
Conflict of interest statement
Figures
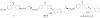
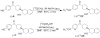
Similar articles
-
Synthesis, resolution, and in vitro evaluation of three vesicular acetylcholine transporter ligands and evaluation of the lead fluorine-18 radioligand in a nonhuman primate.Org Biomol Chem. 2017 Jun 21;15(24):5197-5209. doi: 10.1039/c7ob00854f. Org Biomol Chem. 2017. PMID: 28590490 Free PMC article.
-
Synthesis and evaluation of vesamicol analog (-)-O-[11C]methylvesamicol as a PET ligand for vesicular acetylcholine transporter.Ann Nucl Med. 2006 Jul;20(6):417-24. doi: 10.1007/BF03027377. Ann Nucl Med. 2006. PMID: 16922470
-
Automated production of [¹⁸F]VAT suitable for clinical PET study of vesicular acetylcholine transporter.Appl Radiat Isot. 2016 Jan;107:40-46. doi: 10.1016/j.apradiso.2015.09.010. Epub 2015 Sep 10. Appl Radiat Isot. 2016. PMID: 26408913 Free PMC article.
-
PET radioligands for the vesicular transporters for monoamines and acetylcholine.J Labelled Comp Radiopharm. 2013 Mar-Apr;56(3-4):167-71. doi: 10.1002/jlcr.2998. J Labelled Comp Radiopharm. 2013. PMID: 24285322 Review.
-
PET radioligands for the vesicular acetylcholine transporter (VAChT).Curr Top Med Chem. 2010;10(15):1569-83. doi: 10.2174/156802610793176846. Curr Top Med Chem. 2010. PMID: 20583990 Review.
Cited by
-
Synthesis and biological characterization of a promising F-18 PET tracer for vesicular acetylcholine transporter.Bioorg Med Chem. 2015 Aug 1;23(15):4699-4709. doi: 10.1016/j.bmc.2015.05.058. Epub 2015 Jun 5. Bioorg Med Chem. 2015. PMID: 26138195 Free PMC article.
-
In Vivo and In Vitro Characteristics of Radiolabeled Vesamicol Analogs as the Vesicular Acetylcholine Transporter Imaging Agents.Contrast Media Mol Imaging. 2018 Jun 13;2018:4535476. doi: 10.1155/2018/4535476. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 30008624 Free PMC article. Review.
-
Pharmacologic characterizations of a P2X7 receptor-specific radioligand, [11C]GSK1482160 for neuroinflammatory response.Nucl Med Commun. 2017 May;38(5):372-382. doi: 10.1097/MNM.0000000000000660. Nucl Med Commun. 2017. PMID: 28338530 Free PMC article.
-
The Benzoylpiperidine Fragment as a Privileged Structure in Medicinal Chemistry: A Comprehensive Review.Molecules. 2024 Apr 23;29(9):1930. doi: 10.3390/molecules29091930. Molecules. 2024. PMID: 38731421 Free PMC article. Review.
-
Kinetics modeling and occupancy studies of a novel C-11 PET tracer for VAChT in nonhuman primates.Nucl Med Biol. 2016 Feb;43(2):131-9. doi: 10.1016/j.nucmedbio.2015.11.003. Epub 2015 Nov 7. Nucl Med Biol. 2016. PMID: 26872437 Free PMC article.
References
-
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113:1625–1644. - PubMed
-
- Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. - PubMed
-
- Prado VF, Martins-Silva C, de Castro BM, et al. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron. 2006;51:601–612. - PubMed
-
- Rogers GA, Parsons SM, Anderson DC, et al. Synthesis, in vitro acetylcholine-storage-blocking activities, and biological properties of derivatives and analogues of trans-2-(4-phenylpiperidino)cyclohexanol (vesamicol) J Med Chem. 1989;32:1217–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

