Spatial regulation of Aurora A activity during mitotic spindle assembly requires RHAMM to correctly localize TPX2
- PMID: 24875404
- PMCID: PMC4111680
- DOI: 10.4161/cc.29270
Spatial regulation of Aurora A activity during mitotic spindle assembly requires RHAMM to correctly localize TPX2
Abstract
Construction of a mitotic spindle requires biochemical pathways to assemble spindle microtubules and structural proteins to organize these microtubules into a bipolar array. Through a complex with dynein, the receptor for hyaluronan-mediated motility (RHAMM) cross-links mitotic microtubules to provide structural support, maintain spindle integrity, and correctly orient the mitotic spindle. Here, we locate RHAMM to sites of microtubule assembly at centrosomes and non-centrosome sites near kinetochores and demonstrate that RHAMM is required for the activation of Aurora kinase A. Silencing of RHAMM delays the kinetics of spindle assembly, mislocalizes targeting protein for XKlp2 (TPX2), and attenuates the localized activation of Aurora kinase A with a consequent reduction in mitotic spindle length. The RHAMM-TPX2 complex requires a C-terminal basic leucine zipper in RHAMM and a domain that includes the nuclear localization signal in TPX2. Together, our findings identify RHAMM as a critical regulator for Aurora kinase A signaling and suggest that RHAMM ensures bipolar spindle assembly and mitotic progression through the integration of biochemical and structural pathways.
Keywords: Aurora kinase A; RHAMM; TPX2; mitosis; spindle assembly.
Figures
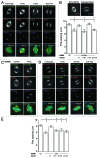
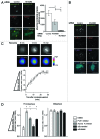
Similar articles
-
CEP192 localises mitotic Aurora-A activity by priming its interaction with TPX2.EMBO J. 2024 Nov;43(22):5381-5420. doi: 10.1038/s44318-024-00240-z. Epub 2024 Sep 26. EMBO J. 2024. PMID: 39327527 Free PMC article.
-
Poleward transport of TPX2 in the mammalian mitotic spindle requires dynein, Eg5, and microtubule flux.Mol Biol Cell. 2010 Mar 15;21(6):979-88. doi: 10.1091/mbc.e09-07-0601. Epub 2010 Jan 28. Mol Biol Cell. 2010. PMID: 20110350 Free PMC article.
-
Overexpression of the receptor for hyaluronan-mediated motility, correlates with expression of microtubule-associated protein in human oral squamous cell carcinomas.Int J Oncol. 2009 Jun;34(6):1565-71. doi: 10.3892/ijo_00000286. Int J Oncol. 2009. PMID: 19424574
-
Role of receptor for hyaluronan-mediated motility (RHAMM) in human head and neck cancers.J Cancer Res Clin Oncol. 2014 Oct;140(10):1629-40. doi: 10.1007/s00432-014-1653-z. Epub 2014 Mar 28. J Cancer Res Clin Oncol. 2014. PMID: 24676428 Free PMC article. Review.
-
Regulation of Aurora-A kinase on the mitotic spindle.Chromosoma. 2003 Dec;112(4):159-63. doi: 10.1007/s00412-003-0265-1. Epub 2003 Nov 21. Chromosoma. 2003. PMID: 14634755 Review.
Cited by
-
Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor.Cells. 2020 Mar 28;9(4):819. doi: 10.3390/cells9040819. Cells. 2020. PMID: 32231069 Free PMC article. Review.
-
RHAMM splice variants confer radiosensitivity in human breast cancer cell lines.Oncotarget. 2016 Apr 19;7(16):21428-40. doi: 10.18632/oncotarget.7258. Oncotarget. 2016. PMID: 26870892 Free PMC article.
-
BRCA1 controls the cell division axis and governs ploidy and phenotype in human mammary cells.Oncotarget. 2017 May 16;8(20):32461-32475. doi: 10.18632/oncotarget.15688. Oncotarget. 2017. PMID: 28427147 Free PMC article.
-
HMMR acts in the PLK1-dependent spindle positioning pathway and supports neural development.Elife. 2017 Oct 10;6:e28672. doi: 10.7554/eLife.28672. Elife. 2017. PMID: 28994651 Free PMC article.
-
RHAMM Is a Multifunctional Protein That Regulates Cancer Progression.Int J Mol Sci. 2021 Sep 24;22(19):10313. doi: 10.3390/ijms221910313. Int J Mol Sci. 2021. PMID: 34638654 Free PMC article. Review.
References
-
- Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, Zhang D, Saya H, Toyoshima YY, Kinoshita K, et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol Cell Biol. 2007;27:352–67. doi: 10.1128/MCB.00878-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
