Venus kinase receptors control reproduction in the platyhelminth parasite Schistosoma mansoni
- PMID: 24875530
- PMCID: PMC4038586
- DOI: 10.1371/journal.ppat.1004138
Venus kinase receptors control reproduction in the platyhelminth parasite Schistosoma mansoni
Erratum in
-
Correction: Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite Schistosoma mansoni.PLoS Pathog. 2016 Jul 25;12(7):e1005798. doi: 10.1371/journal.ppat.1005798. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27454584 Free PMC article.
Abstract
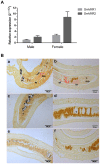
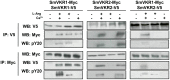
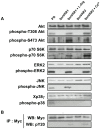
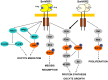
The Venus kinase receptor (VKR) is a single transmembrane molecule composed of an intracellular tyrosine kinase domain close to that of insulin receptor and an extracellular Venus Flytrap (VFT) structure similar to the ligand binding domain of many class C G protein coupled receptors. This receptor tyrosine kinase (RTK) was first discovered in the platyhelminth parasite Schistosoma mansoni, then in a large variety of invertebrates. A single vkr gene is found in most genomes, except in S. mansoni in which two genes Smvkr1 and Smvkr2 exist. VKRs form a unique family of RTKs present only in invertebrates and their biological functions are still to be discovered. In this work, we show that SmVKRs are expressed in the reproductive organs of S. mansoni, particularly in the ovaries of female worms. By transcriptional analyses evidence was obtained that both SmVKRs fulfill different roles during oocyte maturation. Suppression of Smvkr expression by RNA interference induced spectacular morphological changes in female worms with a strong disorganization of the ovary, which was dominated by the presence of primary oocytes, and a defect of egg formation. Following expression in Xenopus oocytes, SmVKR1 and SmVKR2 receptors were shown to be activated by distinct ligands which are L-Arginine and calcium ions, respectively. Signalling analysis in Xenopus oocytes revealed the capacity of SmVKRs to activate the PI3K/Akt/p70S6K and Erk MAPK pathways involved in cellular growth and proliferation. Additionally, SmVKR1 induced phosphorylation of JNK (c-Jun N-terminal kinase). Activation of JNK by SmVKR1 was supported by the results of yeast two-hybrid experiments identifying several components of the JNK pathway as specific interacting partners of SmVKR1. In conclusion, these results demonstrate the functions of SmVKR in gametogenesis, and particularly in oogenesis and egg formation. By eliciting signalling pathways potentially involved in oocyte proliferation, growth and migration, these receptors control parasite reproduction and can therefore be considered as potential targets for anti-schistosome therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Venus Kinase Receptors at the Crossroads of Insulin Signaling: Their Role in Reproduction for Helminths and Insects.Front Endocrinol (Lausanne). 2015 Aug 3;6:118. doi: 10.3389/fendo.2015.00118. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26284029 Free PMC article. Review.
-
SmShb, the SH2-Containing Adaptor Protein B of Schistosoma mansoni Regulates Venus Kinase Receptor Signaling Pathways.PLoS One. 2016 Sep 16;11(9):e0163283. doi: 10.1371/journal.pone.0163283. eCollection 2016. PLoS One. 2016. PMID: 27636711 Free PMC article.
-
Schistosoma mansoni: structural and biochemical characterization of two distinct Venus Kinase Receptors.Exp Parasitol. 2012 Sep;132(1):32-9. doi: 10.1016/j.exppara.2011.05.007. Epub 2011 May 15. Exp Parasitol. 2012. PMID: 21616067
-
Dual targeting of insulin and venus kinase Receptors of Schistosoma mansoni for novel anti-schistosome therapy.PLoS Negl Trop Dis. 2013 May 16;7(5):e2226. doi: 10.1371/journal.pntd.0002226. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23696913 Free PMC article.
-
Venus kinase receptors: prospects in signaling and biological functions of these invertebrate kinases.Front Endocrinol (Lausanne). 2014 May 13;5:72. doi: 10.3389/fendo.2014.00072. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 24860549 Free PMC article. Review.
Cited by
-
Venus Kinase Receptors at the Crossroads of Insulin Signaling: Their Role in Reproduction for Helminths and Insects.Front Endocrinol (Lausanne). 2015 Aug 3;6:118. doi: 10.3389/fendo.2015.00118. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26284029 Free PMC article. Review.
-
Correction: Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite Schistosoma mansoni.PLoS Pathog. 2016 Jul 25;12(7):e1005798. doi: 10.1371/journal.ppat.1005798. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27454584 Free PMC article.
-
Molecular characterization of host-parasite cell signalling in Schistosoma mansoni during early development.Sci Rep. 2016 Oct 20;6:35614. doi: 10.1038/srep35614. Sci Rep. 2016. PMID: 27762399 Free PMC article.
-
Sex-Biased Transcriptome of Schistosoma mansoni: Host-Parasite Interaction, Genetic Determinants and Epigenetic Regulators Are Associated with Sexual Differentiation.PLoS Negl Trop Dis. 2016 Sep 27;10(9):e0004930. doi: 10.1371/journal.pntd.0004930. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27677173 Free PMC article.
-
Glucose Uptake in the Human Pathogen Schistosoma mansoni Is Regulated Through Akt/Protein Kinase B Signaling.J Infect Dis. 2018 Jun 5;218(1):152-164. doi: 10.1093/infdis/jix654. J Infect Dis. 2018. PMID: 29309602 Free PMC article.
References
-
- WHO (2013) Schistosomiasis. WHO Media Centre, Fact sheet N°115. Available: http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed 30 April 2014.
-
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, et al. (2002) Schistosomiasis. N Eng J Med 346: 1212–1220. - PubMed
-
- Doenhoff MJ, Pica-Mattoccia L (2006) Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Exp Rev Anti-Infect Ther 4: 199–210. - PubMed
-
- Pica-Mattoccia L, Doenhoff MJ, Valle C, Basso A, Troiani AR, et al. (2009) Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni . Acta Trop 11: 82–85. - PubMed
-
- Doenhoff MJ, Cioli D, Utzinger J (2008) Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 21: 659–667. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

