Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials
- PMID: 24875610
- PMCID: PMC4151260
- DOI: 10.1001/jamaophthalmol.2014.1019
Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials
Abstract
Importance: Although anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration (AMD) results in improved vision overall, loss of substantial vision can occur. Understanding the processes that lead to loss of vision may lead to preventive strategies.
Objective: To determine the incidence, characteristics, causes, and baseline predictors of sustained visual acuity loss after 2 years of treatment with ranibizumab or bevacizumab for neovascular AMD.
Design, setting, and participants: A cohort study within a randomized clinical trial of participants in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT).
Interventions: Participants were randomly assigned to treatment with ranibizumab or bevacizumab and to 2 years of monthly or as needed injections or monthly injections for 1 year and as needed injections the following year.
Main outcomes and measures: Sustained visual acuity loss, defined as loss of 15 or more letters from baseline at weeks 88 and 104.
Results: Among 1030 participants, 61 eyes (5.9%) developed sustained visual acuity loss in 2 years. Within this group, visual acuity decreased gradually over time, with a mean decrease of 2, 19, and 33 letters from baseline at 4 weeks, 1 year, and 2 years, respectively. At 2 years, eyes with sustained visual acuity loss had more scarring (60.0% vs 41.4%, P = .007), more geographic atrophy (GA) (31.6% vs 20.7%, P = .004), larger lesions (16 vs 8 mm2, P < .001), and higher proportions of intraretinal fluid (82.5% vs 51.0%, P < .001), subretinal hyperreflective material (84.5% vs 44.2%, P < .001), retinal thinning (43.3% vs 23.0%, P < .001), and thickening (20.0% vs 12.1%, P < .001). Likely causes of sustained visual acuity loss included foveal scarring (44.3%), pigmentary abnormalities (27.9%), and foveal GA (11.5%). Baseline factors independently associated with a higher incidence of sustained visual acuity loss were the presence of nonfoveal GA (odds ratio [OR], 2.86; 95% CI, 1.35-6.08; P = .006), larger area of choroidal neovascularization (OR for a >4-disc area vs ≤1-disc area, 3.91; 95% CI, 1.70-9.03; P = .007), and bevacizumab treatment (OR, 1.83; 95% CI, 1.07-3.14; P = .03).
Conclusions and relevance: Sustained visual acuity loss was relatively rare in CATT. The development of foveal scar, pigmentary abnormalities, or GA contributed to most of the sustained visual acuity loss. Risk was 3% higher among eyes treated with bevacizumab. Treatment that targeted the prevention of scarring or GA may improve vision outcomes.
Trial registration: clinicaltrials.gov Identifier: NCT00593450.
Conflict of interest statement
Figures
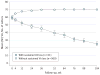
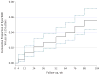
Comment in
-
Analyses Comparing Visual Acuity Between Ranibizumab and Bevacizumab in the Comparison of Age-Related Macular Degeneration Treatments Trials.JAMA Ophthalmol. 2015 Jun;133(6):726. doi: 10.1001/jamaophthalmol.2015.0502. JAMA Ophthalmol. 2015. PMID: 25811288 Free PMC article. No abstract available.
References
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. - PubMed
-
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. e5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

