Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma
- PMID: 24876103
- PMCID: PMC4134724
- DOI: 10.1158/0008-5472.CAN-14-0680
Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma
Abstract
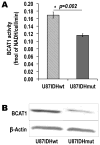
Mutations of the isocitrate dehydrogenase 1 (IDH1) gene are among the most prevalent in low-grade glioma and secondary glioblastoma, represent an early pathogenic event, and are associated with epigenetically driven modulations of metabolism. Of particular interest is the recently uncovered relationship between the IDH1 mutation and decreased activity of the branched-chain amino acid transaminase 1 (BCAT1) enzyme. Noninvasive imaging methods that can assess BCAT1 activity could therefore improve detection of mutant IDH1 tumors and aid in developing and monitoring new targeted therapies. BCAT1 catalyzes the transamination of branched-chain amino acids while converting α-ketoglutarate (α-KG) to glutamate. Our goal was to use (13)C magnetic resonance spectroscopy to probe the conversion of hyperpolarized [1-(13)C] α-KG to hyperpolarized [1-(13)C] glutamate as a readout of BCAT1 activity. We investigated two isogenic glioblastoma lines that differed only in their IDH1 status and performed experiments in live cells and in vivo in rat orthotopic tumors. Following injection of hyperpolarized [1-(13)C] α-KG, hyperpolarized [1-(13)C] glutamate production was detected both in cells and in vivo, and the level of hyperpolarized [1-(13)C] glutamate was significantly lower in mutant IDH1 cells and tumors compared with their IDH1-wild-type counterparts. Importantly however, in our cells the observed drop in hyperpolarized [1-(13)C] glutamate was likely mediated not only by a drop in BCAT1 activity, but also by reductions in aspartate transaminase and glutamate dehydrogenase activities, suggesting additional metabolic reprogramming at least in our model. Hyperpolarized [1-(13)C] glutamate could thus inform on multiple mutant IDH1-associated metabolic events that mediate reduced glutamate production.
©2014 American Association for Cancer Research.
Figures
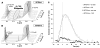
 ) perfused cells, showing the significantly higher level of glutamate in U87IDHwt cells as compared to U87IDHmut. Also of importance is the delayed formation of hyperpolarized [1-13C] glutamate versus the time of maximum hyperpolarized [1-13C] α-KG (vertical dashed line), as expected when metabolism occurs. The fit derived from the gamma-variate analysis (GVA) is displayed as a continuous line for U87IDHwt and as a dashed line for U87IDHmut perfused cells.
) perfused cells, showing the significantly higher level of glutamate in U87IDHwt cells as compared to U87IDHmut. Also of importance is the delayed formation of hyperpolarized [1-13C] glutamate versus the time of maximum hyperpolarized [1-13C] α-KG (vertical dashed line), as expected when metabolism occurs. The fit derived from the gamma-variate analysis (GVA) is displayed as a continuous line for U87IDHwt and as a dashed line for U87IDHmut perfused cells.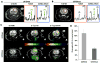

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

