Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy
- PMID: 24876114
- PMCID: PMC4214522
- DOI: 10.1681/ASN.2013080901
Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy
Abstract
Diabetes is associated with activation of the polyol pathway, in which glucose is converted to sorbitol by aldose reductase. Previous studies focused on the role of sorbitol in mediating diabetic complications. However, in the proximal tubule, sorbitol can be converted to fructose, which is then metabolized largely by fructokinase, also known as ketohexokinase, leading to ATP depletion, proinflammatory cytokine expression, and oxidative stress. We and others recently identified a potential deleterious role of dietary fructose in the generation of tubulointerstitial injury and the acceleration of CKD. In this study, we investigated the potential role of endogenous fructose production, as opposed to dietary fructose, and its metabolism through fructokinase in the development of diabetic nephropathy. Wild-type mice with streptozotocin-induced diabetes developed proteinuria, reduced GFR, and renal glomerular and proximal tubular injury. Increased renal expression of aldose reductase; elevated levels of renal sorbitol, fructose, and uric acid; and low levels of ATP confirmed activation of the fructokinase pathway. Furthermore, renal expression of inflammatory cytokines with macrophage infiltration was prominent. In contrast, diabetic fructokinase-deficient mice demonstrated significantly less proteinuria, renal dysfunction, renal injury, and inflammation. These studies identify fructokinase as a novel mediator of diabetic nephropathy and document a novel role for endogenous fructose production, or fructoneogenesis, in driving renal disease.
Copyright © 2014 by the American Society of Nephrology.
Figures
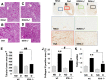
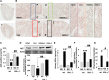
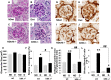



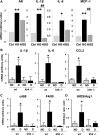
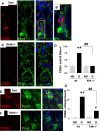
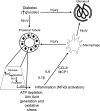
Comment in
-
Sweet debate: fructose versus glucose in diabetic kidney disease.J Am Soc Nephrol. 2014 Nov;25(11):2386-8. doi: 10.1681/ASN.2014050433. Epub 2014 May 29. J Am Soc Nephrol. 2014. PMID: 24876119 Free PMC article. No abstract available.
References
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 - PubMed
-
- Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T: Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 293: F1256–F1261, 2007 - PubMed
-
- Burch HB, Choi S, Dence CN, Alvey TR, Cole BR, Lowry OH: Metabolic effects of large fructose loads in different parts of the rat nephron. J Biol Chem 255: 8239–8244, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

