Rab5a is required for spindle length control and kinetochore-microtubule attachment during meiosis in oocytes
- PMID: 24876181
- PMCID: PMC5395731
- DOI: 10.1096/fj.14-250886
Rab5a is required for spindle length control and kinetochore-microtubule attachment during meiosis in oocytes
Abstract
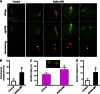
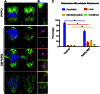
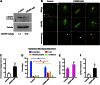
Rab GTPases are highly conserved components of vesicle trafficking pathways. Rab5, as a master regulator of endocytic trafficking, has been shown to function in membrane tethering and docking. However, the function of Rab5 in meiosis has not been addressed. Here, we report elongated spindles and misaligned chromosomes, with kinetochore-microtubule misattachments, on specific depletion of Rab5a in mouse oocytes. Moreover, the localization and levels of centromere protein F (CENPF), a component of the nuclear matrix, are severely reduced at kinetochores in metaphase oocytes following Rab5a knockdown. Consistent with this finding, nuclear lamina disassembly in the transition from prophase arrest to meiosis I is also impaired in Rab5a-depleted oocytes. Notably, oocyte-specific ablation of CENPF phenocopies the meiotic defects resulting from Rab5a knockdown. In summary, our data support a model where Rab5a-positive vesicles, likely through interaction with nuclear lamina, modulate CENPF localization and levels at centromeres, consequently ensuring proper spindle length and kinetochore-microtubule attachment in meiotic oocytes.
Keywords: CENPF; chromosome; vesicles.
© FASEB.
Figures






Similar articles
-
Meiotic Kinetochores Fragment into Multiple Lobes upon Cohesin Loss in Aging Eggs.Curr Biol. 2019 Nov 18;29(22):3749-3765.e7. doi: 10.1016/j.cub.2019.09.006. Epub 2019 Oct 31. Curr Biol. 2019. PMID: 31679939 Free PMC article.
-
Intra-oocyte localization of MAD2 and its relationship with kinetochores, microtubules, and chromosomes in rat oocytes during meiosis.Biol Reprod. 2004 Sep;71(3):740-8. doi: 10.1095/biolreprod.104.028282. Epub 2004 Apr 28. Biol Reprod. 2004. PMID: 15115722
-
CENP-W regulates kinetochore-microtubule attachment and meiotic progression of mouse oocytes.Biochem Biophys Res Commun. 2020 Jun 18;527(1):8-14. doi: 10.1016/j.bbrc.2020.04.078. Epub 2020 Apr 24. Biochem Biophys Res Commun. 2020. PMID: 32446395
-
Aneuploidy in human eggs: contributions of the meiotic spindle.Biochem Soc Trans. 2021 Feb 26;49(1):107-118. doi: 10.1042/BST20200043. Biochem Soc Trans. 2021. PMID: 33449109 Free PMC article. Review.
-
Oocyte Meiotic Spindle Assembly and Function.Curr Top Dev Biol. 2016;116:65-98. doi: 10.1016/bs.ctdb.2015.11.031. Epub 2016 Jan 23. Curr Top Dev Biol. 2016. PMID: 26970614 Free PMC article. Review.
Cited by
-
Cohesin acetyltransferase Esco2 regulates SAC and kinetochore functions via maintaining H4K16 acetylation during mouse oocyte meiosis.Nucleic Acids Res. 2017 Sep 19;45(16):9388-9397. doi: 10.1093/nar/gkx563. Nucleic Acids Res. 2017. PMID: 28934466 Free PMC article.
-
CENP-F-dependent DRP1 function regulates APC/C activity during oocyte meiosis I.Nat Commun. 2022 Dec 13;13(1):7732. doi: 10.1038/s41467-022-35461-5. Nat Commun. 2022. PMID: 36513638 Free PMC article.
-
Involvement of Rab6a in organelle rearrangement and cytoskeletal organization during mouse oocyte maturation.Sci Rep. 2016 Mar 31;6:23560. doi: 10.1038/srep23560. Sci Rep. 2016. PMID: 27030207 Free PMC article.
-
Age-associated accumulation of RAB9 disrupts oocyte meiosis.Aging Cell. 2025 Apr;24(4):e14449. doi: 10.1111/acel.14449. Epub 2024 Dec 15. Aging Cell. 2025. PMID: 39676221 Free PMC article.
-
Loss of CENPF leads to developmental failure in mouse embryos.Cell Cycle. 2019 Oct;18(20):2784-2799. doi: 10.1080/15384101.2019.1661173. Epub 2019 Sep 3. Cell Cycle. 2019. PMID: 31478449 Free PMC article.
References
-
- Krisher R. L. (2004) The effect of oocyte quality on development. J. Animal Sci. 82(E-Suppl.), E14–E23 - PubMed
-
- Hassold T., Hunt P. (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 - PubMed
-
- Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

