Biaxial tensile tests identify epidermis and hypodermis as the main structural elements of sweet cherry skin
- PMID: 24876301
- PMCID: PMC4038440
- DOI: 10.1093/aobpla/plu019
Biaxial tensile tests identify epidermis and hypodermis as the main structural elements of sweet cherry skin
Abstract
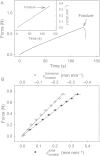
The skin of developing soft and fleshy fruit is subjected to considerable growth stress, and failure of the skin is associated with impaired barrier properties in water transport and pathogen defence. The objectives were to establish a standardized, biaxial tensile test of the skin of soft and fleshy fruit and to use it to characterize and quantify mechanical properties of the sweet cherry (Prunus avium) fruit skin as a model. A segment of the exocarp (ES) comprising cuticle, epidermis, hypodermis and adhering flesh was mounted in the elastometer such that the in vivo strain was maintained. The ES was pressurized from the inner surface and the pressure and extent of associated bulging were recorded. Pressure : strain responses were almost linear up to the point of fracture, indicating that the modulus of elasticity was nearly constant. Abrading the cuticle decreased the fracture strain but had no effect on the fracture pressure. When pressure was held constant, bulging of the ES continued to increase. Strain relaxation upon releasing the pressure was complete and depended on time. Strains in longitudinal and latitudinal directions on the bulging ES did not differ significantly. Exocarp segments that released their in vivo strain before the test had higher fracture strains and lower moduli of elasticity. The results demonstrate that the cherry skin is isotropic in the tangential plane and exhibits elastic and viscoelastic behaviour. The epidermis and hypodermis, but not the cuticle, represent the structural 'backbone' in a cherry skin. This test is useful in quantifying the mechanical properties of soft and fleshy fruit of a range of species under standardized conditions.
Keywords: Biomechanics; Prunus avium; fracture; mechanical properties; rheology; skin; stiffness.; strain.
Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures








References
-
- Bargel H, Spatz HC, Speck T, Neinhuis C. Two-dimensional tension test in plant biomechanics—sweet cherry fruit skin as a model system. Plant Biology. 2004;6:432–439. - PubMed
-
- Chanliaud E, Burrows KM, Jeronomidis G, Gidley MJ. Mechanical properties of primary plant cell wall analogues. Planta. 2002;215:989–996. - PubMed
-
- Cosgrove DJ. Wall extensibility—its nature, measurement and relationship to plant-cell growth. New Phytologist. 1993;124:1–23. - PubMed
-
- Dominguez E, Heredia-Guerrero JA, Heredia A. The biophysical design of plant cuticles: an overview. New Phytologist. 2011a;189:938–949. - PubMed
-
- Dominguez E, Cuartero J, Heredia A. An overview on plant cuticle biomechanics. Plant Science. 2011b;181:77–84. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

