Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise
- PMID: 24876356
- PMCID: PMC4971896
- DOI: 10.1152/japplphysiol.00044.2014
Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise
Abstract
Background: phosphorylation of AS160 and TBC1D1 plays an important role for GLUT4 mobilization to the cell surface. The phosphorylation of AS160 and TBC1D1 in humans in response to acute exercise is not fully characterized.
Objective: to study AS160 and TBC1D1 phosphorylation in human skeletal muscle after aerobic exercise followed by a hyperinsulinemic euglycemic clamp.
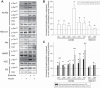
Design: eight healthy men were studied on two occasions: 1) in the resting state and 2) in the hours after a 1-h bout of ergometer cycling. A hyperinsulinemic euglycemic clamp was initiated 240 min after exercise and in a time-matched nonexercised control condition. We obtained muscle biopsies 30 min after exercise and in a time-matched nonexercised control condition (t = 30) and after 30 min of insulin stimulation (t = 270) and investigated site-specific phosphorylation of AS160 and TBC1D1.
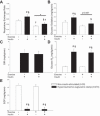
Results: phosphorylation on AS160 and TBC1D1 was increased 30 min after the exercise bout, whereas phosphorylation of the putative upstream kinases, Akt and AMPK, was unchanged compared with resting control condition. Exercise augmented insulin-stimulated phosphorylation on AS160 at Ser(341) and Ser(704) 270 min after exercise. No additional exercise effects were observed on insulin-stimulated phosphorylation of Thr(642) and Ser(588) on AS160 or Ser(237) and Thr(596) on TBC1D1.
Conclusions: AS160 and TBC1D1 phosphorylations were evident 30 min after exercise without simultaneously increased Akt and AMPK phosphorylation. Unlike TBC1D1, insulin-stimulated site-specific AS160 phosphorylation is modified by prior exercise, but these sites do not include Thr(642) and Ser(588). Together, these data provide new insights into phosphorylation of key regulators of glucose transport in human skeletal muscle.
Keywords: AS160; TBC1D1; TBC1D4; endurance exercise; insulin sensitivity; skeletal muscle.
Copyright © 2014 the American Physiological Society.
Figures




Similar articles
-
Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle.Diabetologia. 2015 Jan;58(1):19-30. doi: 10.1007/s00125-014-3395-5. Epub 2014 Oct 4. Diabetologia. 2015. PMID: 25280670 Free PMC article. Review.
-
Exercise increases TBC1D1 phosphorylation in human skeletal muscle.Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E164-71. doi: 10.1152/ajpendo.00042.2011. Epub 2011 Apr 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21505148 Free PMC article. Clinical Trial.
-
Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle.Diabetes. 2009 May;58(5):1096-104. doi: 10.2337/db08-1477. Epub 2009 Feb 10. Diabetes. 2009. PMID: 19208911 Free PMC article.
-
Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle.Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E242-51. doi: 10.1152/ajpendo.00194.2009. Epub 2009 May 12. Am J Physiol Endocrinol Metab. 2009. PMID: 19435856 Free PMC article.
-
Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
Cited by
-
Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle.Diabetologia. 2015 Jan;58(1):19-30. doi: 10.1007/s00125-014-3395-5. Epub 2014 Oct 4. Diabetologia. 2015. PMID: 25280670 Free PMC article. Review.
-
Exercise-induced increase in muscle insulin sensitivity in men is amplified when assessed using a meal test.Diabetologia. 2024 Jul;67(7):1386-1398. doi: 10.1007/s00125-024-06148-x. Epub 2024 Apr 25. Diabetologia. 2024. PMID: 38662135 Free PMC article.
-
Exercise and Omentin: Their Role in the Crosstalk Between Muscle and Adipose Tissues in Type 2 Diabetes Mellitus Rat Models.Front Physiol. 2019 Jan 7;9:1881. doi: 10.3389/fphys.2018.01881. eCollection 2018. Front Physiol. 2019. PMID: 30666216 Free PMC article.
-
Sex differences in the metabolism of glucose and fatty acids by adipose tissue and skeletal muscle in humans.Physiol Rev. 2025 Jul 1;105(3):897-934. doi: 10.1152/physrev.00008.2024. Epub 2025 Jan 27. Physiol Rev. 2025. PMID: 39869194 Review.
-
Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise.Am J Physiol Endocrinol Metab. 2015 Dec 15;309(12):E949-59. doi: 10.1152/ajpendo.00416.2015. Epub 2015 Oct 20. Am J Physiol Endocrinol Metab. 2015. PMID: 26487009 Free PMC article. Review.
References
-
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. - PubMed
-
- Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20: 586–590, 1974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

