Mammalian COPII coat component SEC24C is required for embryonic development in mice
- PMID: 24876386
- PMCID: PMC4110293
- DOI: 10.1074/jbc.M114.566687
Mammalian COPII coat component SEC24C is required for embryonic development in mice
Abstract
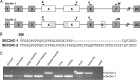
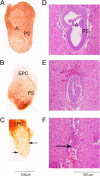
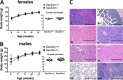
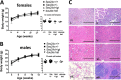
COPII-coated vesicles mediate the transport of newly synthesized proteins from the endoplasmic reticulum to the Golgi. SEC24 is the COPII component primarily responsible for recruitment of protein cargoes into nascent vesicles. There are four Sec24 paralogs in mammals, with mice deficient in SEC24A, -B, and -D exhibiting a wide range of phenotypes. We now report the characterization of mice with deficiency in the fourth Sec24 paralog, SEC24C. Although mice haploinsufficient for Sec24c exhibit no apparent abnormalities, homozygous deficiency results in embryonic lethality at approximately embryonic day 7. Tissue-specific deletion of Sec24c in hepatocytes, pancreatic cells, smooth muscle cells, and intestinal epithelial cells results in phenotypically normal mice. Thus, SEC24C is required in early mammalian development but is dispensable in a number of tissues, likely as a result of compensation by other Sec24 paralogs. The embryonic lethality resulting from loss of SEC24C occurs considerably later than the lethality previously observed in SEC24D deficiency; it is clearly distinct from the restricted neural tube phenotype of Sec24b null embryos and the mild hypocholesterolemic phenotype of adult Sec24a null mice. Taken together, these results demonstrate that the four Sec24 paralogs have developed unique functions over the course of vertebrate evolution.
Figures






References
-
- Bonifacino J. S., Glick B. S. (2004) The mechanisms of vesicle budding and fusion. Cell 116, 153–166 - PubMed
-
- Palade G. (1975) Intracellular aspects of the process of protein synthesis. Science 189, 867–867 - PubMed
-
- Budnik A., Stephens D. J. (2009) ER exit sites–localization and control of COPII vesicle formation. FEBS Lett. 583, 3796–3803 - PubMed
-
- Lee M. C., Miller E. A. (2007) Molecular mechanisms of COPII vesicle formation. Semin. Cell Dev. Biol. 18, 424–434 - PubMed
-
- Zanetti G., Pahuja K. B., Studer S., Shim S., Schekman R. (2012) COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 14, 20–28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

