Inward cholesterol gradient of the membrane system in P. falciparum-infected erythrocytes involves a dilution effect from parasite-produced lipids
- PMID: 24876390
- PMCID: PMC4058088
- DOI: 10.1242/bio.20147732
Inward cholesterol gradient of the membrane system in P. falciparum-infected erythrocytes involves a dilution effect from parasite-produced lipids
Abstract
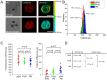
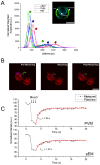
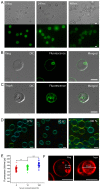
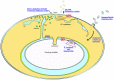
Plasmodium falciparum (Pf) infection remodels the human erythrocyte with new membrane systems, including a modified host erythrocyte membrane (EM), a parasitophorous vacuole membrane (PVM), a tubulovesicular network (TVN), and Maurer's clefts (MC). Here we report on the relative cholesterol contents of these membranes in parasitized normal (HbAA) and hemoglobin S-containing (HbAS, HbAS) erythrocytes. Results from fluorescence lifetime imaging microscopy (FLIM) experiments with a cholesterol-sensitive fluorophore show that membrane cholesterol levels in parasitized erythrocytes (pRBC) decrease inwardly from the EM, to the MC/TVN, to the PVM, and finally to the parasite membrane (PM). Cholesterol depletion of pRBC by methyl-β-cyclodextrin treatment caused a collapse of this gradient. Lipid and cholesterol exchange data suggest that the cholesterol gradient involves a dilution effect from non-sterol lipids produced by the parasite. FLIM signals from the PVM or PM showed little or no difference between parasitized HbAA vs HbS-containing erythrocytes that differed in lipid content, suggesting that malaria parasites may regulate the cholesterol contents of the PVM and PM independently of levels in the host cell membrane. Cholesterol levels may affect raft structures and the membrane trafficking and sorting functions that support Pf survival in HbAA, HbAS and HbSS erythrocytes.
Keywords: Detergent-resistant membrane domain; Fluorescence lifetime imaging microscopy; Malaria; Maurer's cleft; Membrane rafts; Parasitophorous vacuole membrane; Plasmodium falciparum.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures




Similar articles
-
Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes.Mol Cell Biol. 2006 Jun;26(11):4074-85. doi: 10.1128/MCB.00095-06. Mol Cell Biol. 2006. PMID: 16705161 Free PMC article.
-
Distribution of malaria parasite-derived phosphatidylcholine in the infected erythrocyte.mSphere. 2023 Oct 24;8(5):e0013123. doi: 10.1128/msphere.00131-23. Epub 2023 Aug 22. mSphere. 2023. PMID: 37606582 Free PMC article.
-
Electron tomography of the Maurer's cleft organelles of Plasmodium falciparum-infected erythrocytes reveals novel structural features.Mol Microbiol. 2008 Feb;67(4):703-18. doi: 10.1111/j.1365-2958.2007.06063.x. Epub 2007 Dec 7. Mol Microbiol. 2008. PMID: 18067543
-
Remodeling of human red cells infected with Plasmodium falciparum and the impact of PHIST proteins.Blood Cells Mol Dis. 2013 Oct;51(3):195-202. doi: 10.1016/j.bcmd.2013.06.003. Epub 2013 Jul 21. Blood Cells Mol Dis. 2013. PMID: 23880461 Review.
-
Protein and lipid trafficking induced in erythrocytes infected by malaria parasites.Cell Microbiol. 2002 Jul;4(7):383-95. doi: 10.1046/j.1462-5822.2002.00204.x. Cell Microbiol. 2002. PMID: 12102685 Review.
Cited by
-
A comparative study of the localization and membrane topology of members of the RIFIN, STEVOR and PfMC-2TM protein families in Plasmodium falciparum-infected erythrocytes.Malar J. 2015 Jul 16;14:274. doi: 10.1186/s12936-015-0784-2. Malar J. 2015. PMID: 26173856 Free PMC article.
-
Diverse Chemical Compounds Target Plasmodium falciparum Plasma Membrane Lipid Homeostasis.ACS Infect Dis. 2019 Apr 12;5(4):550-558. doi: 10.1021/acsinfecdis.8b00277. Epub 2019 Jan 28. ACS Infect Dis. 2019. PMID: 30638365 Free PMC article.
-
Polyunsaturated fatty acids promote Plasmodium falciparum gametocytogenesis.Biol Open. 2019 Jul 16;8(7):bio042259. doi: 10.1242/bio.042259. Biol Open. 2019. PMID: 31221627 Free PMC article.
-
Hardly Vacuous: The Parasitophorous Vacuolar Membrane of Malaria Parasites.Trends Parasitol. 2020 Feb;36(2):138-146. doi: 10.1016/j.pt.2019.11.006. Epub 2019 Dec 19. Trends Parasitol. 2020. PMID: 31866184 Free PMC article. Review.
-
γδ T Cells Kill Plasmodium falciparum in a Granzyme- and Granulysin-Dependent Mechanism during the Late Blood Stage.J Immunol. 2020 Apr 1;204(7):1798-1809. doi: 10.4049/jimmunol.1900725. Epub 2020 Feb 17. J Immunol. 2020. PMID: 32066596 Free PMC article.
References
-
- Aikawa M. (1988). Morphological changes in erythrocytes induced by malarial parasites. Biol. Cell 64, 173–181. - PubMed
-
- Aikawa M., Uni Y., Andrutis A. T., Howard R. J. (1986). Membrane-associated electron-dense material of the asexual stages of Plasmodium falciparum: evidence for movement from the intracellular parasite to the erythrocyte membrane. Am. J. Trop. Med. Hyg. 35, 30–36. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

