Application of PK/PD modeling and simulation to dosing regimen optimization of high-dose human regular U-500 insulin
- PMID: 24876428
- PMCID: PMC4764242
- DOI: 10.1177/1932296814532326
Application of PK/PD modeling and simulation to dosing regimen optimization of high-dose human regular U-500 insulin
Abstract
Pharmacokinetic/pharmacodynamic (PK/PD) studies of human regular U-500 insulin (U-500R) at high doses commonly used in clinical practice (>100 units) have not been performed. The current analysis applied PK/PD modeling/simulation to fit the data and simulate single-dose and steady-state PK/PD of U-500R high-dose regimens. Data from 3 single-dose euglycemic clamp studies in healthy obese and normal-weight patients, and normal-weight patients with type 1 diabetes were used to build the model. The model was sequential (PK inputs fed into PD component). PK was described using a 1-compartment model with first-order absorption and elimination. The model estimated separate absorption rate constants for U-500R and human regular U-100 insulin. The PD component used an effect compartment model, parameterized in terms of maximum pharmacologic effect (E(max)) and concentration to achieve 50% of E(max). The model described the data well. Steady-state PK for once-daily (QD), twice-daily (BID), or thrice-daily (TID) administration appeared to be reached 24 hours after the first dose. At steady-state, QD dosing showed the greatest fluctuations in PK/PD. BID dosing showed a gradual increase in insulin action with each dose and a fairly stable basal insulin effect. For TID dosing, activity was maintained throughout the dosing interval. PK/PD modeling/simulation of high U-500R doses supports BID or TID administration with an extended duration of activity relative to QD. TID dosing may provide slightly better full-day insulin effect. Additional PK/PD studies and randomized controlled trials of U-500R are needed to validate model predictions in patients with insulin-resistant diabetes requiring high-dose insulin.
Keywords: U-500 regular human insulin; dosing; modeling; pharmacodynamics; pharmacokinetics; simulation; time action profile.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures

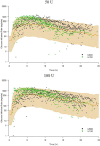
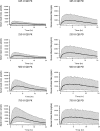
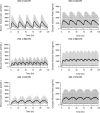
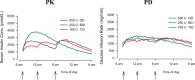

References
-
- Humulin R® U-500 [product label]. Indianapolis, IN: Eli Lilly and Company; 2013.
-
- NIH Clinical Trials Study Record Detail. U.S. National Institutes of Health website. Available at: http://clinicaltrials.gov/ct2/show/NCT01774968. NLM Identifier: NCT01774968. Accessed July 21, 2013.
-
- IMS Health National Prescription Audit Monthly, January 2007-August 2010. Data on file. Indianapolis, IN: Eli Lilly and Company.
-
- Galloway JA, Spradlin CT, Nelson RL, Wentworth SM, Davidson JA, Swarner JL. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981;4:366-376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

