Feasibility of Factory Calibration for Subcutaneous Glucose Sensors in Subjects With Diabetes
- PMID: 24876543
- PMCID: PMC4454101
- DOI: 10.1177/1932296813511747
Feasibility of Factory Calibration for Subcutaneous Glucose Sensors in Subjects With Diabetes
Abstract
Background: Continuous glucose monitoring using subcutaneously inserted sensors currently requires blood glucose tests for sensor calibration. Alternatively, sensors precalibrated during the manufacturing process may eliminate the need for fingerstick calibrations. In this study we evaluated the feasibility of sensor factory calibration in subjects with diabetes.
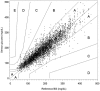
Methods: A total of 33 subjects with diabetes were asked to wear 4 sensors in parallel, 2 on the arm and 2 on the abdomen. Sensors from a lot with low in vitro sensitivity coefficient of variation were used in the study. Based on frequent capillary blood glucose measurements, the average glucose sensitivity of each sensor was determined over a 5-day wear time. The in vivo sensitivities were analyzed for inter- and intrasubject variation. Mean absolute relative difference (MARD) calculation and consensus error grid analysis (EGA) were performed using a single calibration factor for all sensors, to simulate factory calibration and compared against conventional finger-stick calibration.
Results: The sensitivity coefficient of variation between sensors increased from 2.9% in vitro to 6.0% in vivo. No difference in sensor response between subjects (P = .069) as well as between insertion sites (arm and abdomen) was detected (P = .104). Applying one calibration factor to all sensors in the study resulted in an MARD of 13.4%, and 83.5% of the values fell in consensus EGA zone A. Multiple fingerstick calibration resulted in an MARD of 12.7% and 84.1% in zone A.
Conclusions: Feasibility of factory calibration was demonstrated in subjects with diabetes using sensors based on "wired enzyme" technology, resulting in accuracy metrics similar to sensors calibrated with capillary blood glucose.
Keywords: calibration; continuous glucose monitoring; diabetes; glucose sensor; subcutaneous.
© 2013 Diabetes Technology Society.
Conflict of interest statement
Figures

References
-
- Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius J Anal Chem. 2000;366(6-7):611-621. - PubMed
-
- Hoss U, Jeddi I, Schulz M, et al. Continuous glucose monitoring in subcutaneous tissue using factory-calibrated sensors: a pilot study. Diabetes Technol Ther. 2010;12(8):591-597. - PubMed
-
- Feldman B, Brazg R, Schwartz S, et al. A continuous glucose sensor based on Wired Enzyme™ technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther. 2003;5(5):769-782. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

