Validity and Reliability of a Glucometer Against Industry Reference Standards
- PMID: 24876544
- PMCID: PMC4454112
- DOI: 10.1177/1932296813514315
Validity and Reliability of a Glucometer Against Industry Reference Standards
Abstract
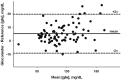
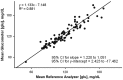
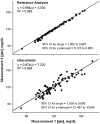
As an appealing alternative to reference glucose analyzers, portable glucometers are recommended for self-monitoring at home, in the field, and in research settings. The purpose was to characterize the accuracy and precision, and bias of glucometers in biomedical research. Fifteen young (20-36 years; mean = 24.5), moderately to highly active men (n = 10) and women (n = 5), defined by exercising 2 to 3 times a week for the past 6 months, were given an oral glucose tolerance test (OGTT) after an overnight fast. Participants ingested 50, 75, or 150 grams of glucose over a 5-minute period. The glucometer was compared to a reference instrument. The glucometer had 39% of values within 15% of measurements made using the reference instrument ranging from 45.05 to 169.37 mg/dl. There was both a proportional (-0.45 to -0.39) and small fixed (5.06 and 0.90 mg/dl) bias. Results of the present study suggest that the glucometer provided poor validity and reliability results compared to the results provided by the reference laboratory analyzer. The portable glucometers should be used for patient management, but not for diagnosis, treatment, or research purposes.
Keywords: accuracy; bias; oral glucose tolerance test; reproducibility.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures
References
-
- American Diabetes Association. Tests of glycemia in diabetes. Diabetes Care. 2003;26(S1):S106-S108. - PubMed
-
- Kimberly MM, Vesper HW, Caudill SP, et al. Variability among five over-the-counter blood glucose monitors. Clin Chim Acta. 2006;364(1-2):292-297. - PubMed
-
- Strok ADM, Kemperman H, Erkelens DW, et al. Comparison of the accuracy of the hemocue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153:275-281. - PubMed
-
- Böhme P, Floriot M, Sirveaux MA, et al. Evolution of analytical performance in portable glucose meters in the last decade. Diabetes Care. 2003;26:1170-1175. - PubMed
-
- Alto WA, Meyer D, Schneid J, et al. Assuring the accuracy of home glucose monitoring. J Am Board Fam Pract. 2002;15:1-6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous