Extracellular Matrix Scaffold Technology for Bioartificial Pancreas Engineering: State of the Art and Future Challenges
- PMID: 24876552
- PMCID: PMC4454093
- DOI: 10.1177/1932296813519558
Extracellular Matrix Scaffold Technology for Bioartificial Pancreas Engineering: State of the Art and Future Challenges
Abstract
Emergent technologies in regenerative medicine may soon overcome the limitations of conventional diabetes therapies. Collaborative efforts across the subfields of stem cell technology, islet encapsulation, and biomaterial carriers seek to produce a bioengineered pancreas capable of restoring endocrine function in patients with insulin-dependent diabetes. These technologies rely on a robust understanding of the extracellular matrix (ECM), the supportive 3-dimensional network of proteins necessary for cellular attachment, proliferation, and differentiation. Although these functions can be partially approximated by biosynthetic carriers, novel decellularization protocols have allowed researchers to discover the advantages afforded by the native pancreatic ECM. The native ECM has proven to be an optimal platform for recellularization and whole-organ pancreas bioengineering, an exciting new field with the potential to resolve the dire shortage of transplantable organs. This review seeks to contextualize recent findings, discuss current research goals, and identify future challenges of regenerative medicine as it applies to diabetes management.
Keywords: bioartificial pancreas; diabetes mellitus; extracellular matrix; insulin; regenerative medicine; stem cells.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures

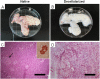
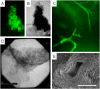

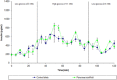
Similar articles
-
Tissue engineering of decellularized pancreas scaffolds for regenerative medicine in diabetes.Acta Biomater. 2023 Feb;157:49-66. doi: 10.1016/j.actbio.2022.11.032. Epub 2022 Nov 24. Acta Biomater. 2023. PMID: 36427686 Review.
-
Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering.Biomaterials. 2013 Jul;34(22):5488-95. doi: 10.1016/j.biomaterials.2013.03.054. Epub 2013 Apr 10. Biomaterials. 2013. PMID: 23583038 Free PMC article.
-
The rat pancreatic body tail as a source of a novel extracellular matrix scaffold for endocrine pancreas bioengineering.J Biol Eng. 2018 Apr 27;12:6. doi: 10.1186/s13036-018-0096-5. eCollection 2018. J Biol Eng. 2018. PMID: 29719565 Free PMC article.
-
Cell replacement strategies aimed at reconstitution of the β-cell compartment in type 1 diabetes.Diabetes. 2014 May;63(5):1433-44. doi: 10.2337/db13-1742. Diabetes. 2014. PMID: 24757193 Review.
-
The Most Ideal Pancreas Extracellular Matrix as a Platform for Pancreas Bioengineering: Decellularization/Recellularization Protocols.Adv Exp Med Biol. 2021;1345:61-70. doi: 10.1007/978-3-030-82735-9_6. Adv Exp Med Biol. 2021. PMID: 34582014 Review.
Cited by
-
Bioengineering for Organ Transplantation: Progress and Challenges.Bioengineered. 2015;6(5):257-61. doi: 10.1080/21655979.2015.1081320. Epub 2015 Aug 11. Bioengineered. 2015. PMID: 26259720 Free PMC article. Review.
-
Unlocking the Promise of Decellularized Pancreatic Tissue: A Novel Approach to Support Angiogenesis in Engineered Tissue.Bioengineering (Basel). 2024 Feb 14;11(2):183. doi: 10.3390/bioengineering11020183. Bioengineering (Basel). 2024. PMID: 38391669 Free PMC article.
-
Biomechanics of Additively Manufactured Metallic Scaffolds-A Review.Materials (Basel). 2021 Nov 12;14(22):6833. doi: 10.3390/ma14226833. Materials (Basel). 2021. PMID: 34832234 Free PMC article. Review.
-
Extracellular Matrix-Associated Factors Play Critical Roles in Regulating Pancreatic β-Cell Proliferation and Survival.Endocrinology. 2019 Aug 1;160(8):1885-1894. doi: 10.1210/en.2019-00206. Endocrinology. 2019. PMID: 31271410 Free PMC article. Review.
-
Therapeutic Strategies for Modulating the Extracellular Matrix to Improve Pancreatic Islet Function and Survival After Transplantation.Curr Diab Rep. 2018 May 19;18(7):39. doi: 10.1007/s11892-018-1014-4. Curr Diab Rep. 2018. PMID: 29779190 Free PMC article. Review.
References
-
- Orlando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2011;16:110-115. - PubMed
-
- The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289-1298. - PubMed
-
- Ruggenenti P, Remuzzi A, Remuzzi G. Decision time for pancreatic islet-cell transplantation. Lancet. 2008;371(9616):883-884. - PubMed
-
- Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318-1330. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

