Design of the Glucose Rate Increase Detector: A Meal Detection Module for the Health Monitoring System
- PMID: 24876583
- PMCID: PMC4455414
- DOI: 10.1177/1932296814523881
Design of the Glucose Rate Increase Detector: A Meal Detection Module for the Health Monitoring System
Abstract
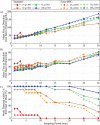
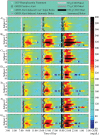
The Glucose Rate Increase Detector (GRID), a module of the Health Monitoring System (HMS), has been designed to operate in parallel to the glucose controller to detect meal events and safely trigger a meal bolus. The GRID algorithm was tuned on clinical data with 40-70 g CHO meals and tested on simulation data with 50-100 g CHO meals. Active closed- and open-loop protocols were executed in silico with various treatments, including automatic boluses based on a 75 g CHO meal and boluses based on simulated user input of meal size. An optional function was used to reduce the recommended bolus using recent insulin and glucose history. For closed-loop control of a 3-meal scenario (50, 75, and 100 g CHO), the GRID improved median time in the 80-180 mg/dL range by 17% and in the >180 range by 14% over unannounced meals, using an automatic bolus for a 75 g CHO meal at detection. Under open-loop control of a 75 g CHO meal, the GRID shifted the median glucose peak down by 73 mg/dL and earlier by 120 min and reduced the time >180 mg/dL by 57% over a missed-meal bolus scenario, using a full meal bolus at detection. The GRID improved closed-loop control in the presence of large meals, without increasing late postprandial hypoglycemia. Users of basal-bolus therapy could also benefit from GRID as a safety alert for missed meal corrections.
Keywords: Health Monitoring System; artificial pancreas; meal detection; safety.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures






References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. - PubMed
-
- Brazeau AS, Mircescu H, Desjardins K, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pract. 2013;99(1):19-23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

