Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor
- PMID: 24876610
- PMCID: PMC4455438
- DOI: 10.1177/1932296814526191
Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor
Abstract
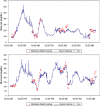
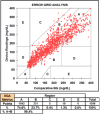
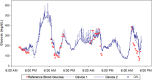
The development of accurate, minimally invasive continuous glucose monitoring (CGM) devices has been the subject of much work by several groups, as it is believed that a less invasive and more user-friendly device will result in greater adoption of CGM by persons with insulin-dependent diabetes. This article presents the results of preliminary clinical studies in subjects with diabetes of a novel prototype microneedle-based continuous glucose monitor. In this device, an array of tiny hollow microneedles is applied into the epidermis from where glucose in interstitial fluid (ISF) is transported via passive diffusion to an amperometric glucose sensor external to the body. Comparison of 1396 paired device glucose measurements and fingerstick blood glucose readings for up to 72-hour wear in 10 diabetic subjects shows the device to be accurate and well tolerated by the subjects. Overall mean absolute relative difference (MARD) is 15% with 98.4% of paired points in the A+B region of the Clarke error grid. The prototype device has demonstrated clinically accurate glucose readings over 72 hours, the first time a microneedle-based device has achieved such performance.
Keywords: continuous glucose; interstitial fluid; microneedle; minimally invasive.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures


Similar articles
-
A human pilot study of the fluorescence affinity sensor for continuous glucose monitoring in diabetes.J Diabetes Sci Technol. 2012 Mar 1;6(2):362-70. doi: 10.1177/193229681200600222. J Diabetes Sci Technol. 2012. PMID: 22538148 Free PMC article.
-
Comparison of Interstitial Fluid Glucose Levels Obtained by Continuous Glucose Monitoring and Flash Glucose Monitoring in Patients With Type 2 Diabetes Mellitus Undergoing Hemodialysis.J Diabetes Sci Technol. 2020 Nov;14(6):1088-1094. doi: 10.1177/1932296819882690. Epub 2019 Oct 18. J Diabetes Sci Technol. 2020. PMID: 31625413 Free PMC article.
-
Accuracy of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System During 10 Days of Use in Youth and Adults with Diabetes.Diabetes Technol Ther. 2018 Jun;20(6):395-402. doi: 10.1089/dia.2018.0150. Epub 2018 Jun 14. Diabetes Technol Ther. 2018. PMID: 29901421 Free PMC article.
-
Delays in minimally invasive continuous glucose monitoring devices: a review of current technology.J Diabetes Sci Technol. 2009 Sep 1;3(5):1207-14. doi: 10.1177/193229680900300528. J Diabetes Sci Technol. 2009. PMID: 20144438 Free PMC article. Review.
-
Use of microneedle array devices for continuous glucose monitoring: a review.Diabetes Technol Ther. 2013 Jan;15(1):101-15. doi: 10.1089/dia.2012.0188. Epub 2012 Dec 12. Diabetes Technol Ther. 2013. PMID: 23234256 Review.
Cited by
-
Where Microneedle Meets Biomarkers: Futuristic Application for Diagnosing and Monitoring Localized External Organ Diseases.Adv Healthc Mater. 2023 Feb;12(5):e2202066. doi: 10.1002/adhm.202202066. Epub 2022 Dec 5. Adv Healthc Mater. 2023. PMID: 36414019 Free PMC article. Review.
-
Hydrogel Microfilaments toward Intradermal Health Monitoring.iScience. 2019 Nov 22;21:328-340. doi: 10.1016/j.isci.2019.10.036. Epub 2019 Oct 23. iScience. 2019. PMID: 31698247 Free PMC article.
-
Epidermal Wearable Biosensors for the Continuous Monitoring of Biomarkers of Chronic Disease in Interstitial Fluid.Micromachines (Basel). 2023 Jul 20;14(7):1452. doi: 10.3390/mi14071452. Micromachines (Basel). 2023. PMID: 37512763 Free PMC article. Review.
-
Flexible Enzymatic Glucose Electrochemical Sensor Based on Polystyrene-Gold Electrodes.Micromachines (Basel). 2021 Jul 7;12(7):805. doi: 10.3390/mi12070805. Micromachines (Basel). 2021. PMID: 34357215 Free PMC article.
-
Point-of-Care Diagnostics: Recent Developments in a Connected Age.Anal Chem. 2017 Jan 3;89(1):102-123. doi: 10.1021/acs.analchem.6b04630. Epub 2016 Dec 13. Anal Chem. 2017. PMID: 27958710 Free PMC article. Review.
References
-
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. - PubMed
-
- DirecNet Study Group. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10(2):91-96. - PubMed
-
- Hover CG, Stedeford T, Vigneulle RM. The development of minimally invasive continuous metabolic monitoring technologies in the U.S. Army TMM Research Program. Diabetes Tech Ther. 2005;7(1):213-224. - PubMed
-
- Tierney MJ, Tamada JA, Potts RO, et al. The GlucoWatch® Biographer: A frequent, automatic and non-invasive glucose monitor. Ann Med. 2000;32:632-641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

