The efficacy and safety of thalidomide-based therapy in patients with advanced non-small cell lung cancer: a meta-analysis
- PMID: 24876820
- PMCID: PMC4037987
- DOI: 10.5114/wo.2014.40782
The efficacy and safety of thalidomide-based therapy in patients with advanced non-small cell lung cancer: a meta-analysis
Abstract
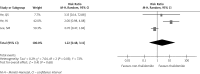
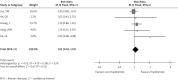
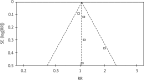
Several randomized controlled clinical trials have compared therapy with or without thalidomide in the treatment of advanced non-small cell lung cancer (NSCLC). However, these studies did not produce consistent results. We carried out a meta-analysis to determine the efficacy and safety of thalidomide-based therapy in patients with advanced NSCLC. For this meta-analysis, we selected randomized clinical trials that compared thalidomide in combination with other therapy or other therapy alone in patients with advanced NSCLC. The outcomes included median overall survival (OS), one- and two-year survival, tumor response, and toxicities. Hazard ratios (HRs) or risk ratios (RRs) were reported with 95% confidence intervals (CIs). A total of 5 eligible trials were included for the meta-analysis, with 729 patients in the thalidomide group and 711 patients in the control group. Compared with non-thalidomide-based therapy, patients receiving thalidomide plus other therapy did not differ significantly in terms of one- and two-year survival or tumor response (RR = 1.32, 95% CI: 0.66-2.63, p = 0.43; RR = 1.22, 95% CI: 0.48-3.11, p = 0.68; RR = 1.05, 95% CI: 0.92-1.19, p = 0.51, respectively). However, thalidomide-based therapy induced more grade 3-4 dizziness and constipation (RR = 2.05, 95% CI: 1.10-3.81, p = 0.02; RR = 4.78, 95% CI: 1.84-12.38, p = 0.001, respectively). The addition of thalidomide to other therapy did not improve survival and tumor response in patients with advanced NSCLC, and thalidomide-based therapy was associated with more grade 3/4 dizziness and constipation.
Keywords: carcinoma; meta-analysis; non-small cell lung; thalidomide.
Figures





References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. - PubMed
-
- Pérol M, Arpin D. Angiogenesis and lung cancer. Bull Cancer. 2007;94:S220–S31. Spec No. - PubMed
-
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase iii study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage iii non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–9. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
