Potential retinoid x receptor agonists for treating Alzheimer's disease from traditional chinese medicine
- PMID: 24876869
- PMCID: PMC4021742
- DOI: 10.1155/2014/278493
Potential retinoid x receptor agonists for treating Alzheimer's disease from traditional chinese medicine
Abstract
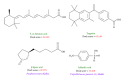
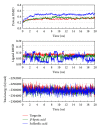
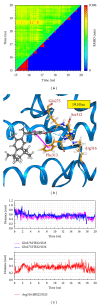
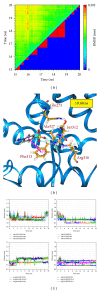
Alzheimer's disease is neurodegenerative disorder due to the accumulation of amyloid- β in the brain and causes dementia with ageing. Some researches indicate that the RXR agonist, Targretin, has also been used for treatment of Alzheimer's disease in mouse models. We investigate the potent candidates as RXR agonists from the vast repertoire of TCM compounds in TCM Database@Taiwan. The potential TCM compounds, β -lipoic acid and sulfanilic acid, had higher potent binding affinities than both 9-cis-retinoic acid and Targretin in docking simulation and have stable H-bonds with residues Arg316 and some equivalent hydrophobic contacts with residues Ala272, Gln275, Leu309, Phe313, Val342, Ile345, and Cys432 as Targretin. The carboxyl or sulfonyl hydroxide group can form a H-bond with key residue Arg316 in the docking pose, and the phenyl group next to the carboxyl or sulfonyl hydroxide group can form a π interaction with residue Phe313. Moreover, β -lipoic acid and sulfanilic acid have stable H-bonds with residue Gln275, Ser313, and residue Ala327, respectively, which may strengthen and stabilize TCM candidates inside the binding domain of RXR protein. Hence, we propose β -lipoic acid and sulfanilic acid as potential lead compounds for further study in drug development process with the RXR protein against Alzheimer's disease.
Figures






References
-
- Crunkhorn S. Neurodegenerative disease: RXR agonist reverses Alzheimer’s disease. Nature Reviews Drug Discovery. 2012;11(4):p. 271. - PubMed
-
- LaFerla FM. Preclinical Success against Alzheimer's Disease with an old drug. New England Journal of Medicine. 2012;367(6):570–572. - PubMed
-
- Aicardi G. New hope from an old drug: fighting Alzheimer's disease with the cancer drug bexarotene (targretin)? Rejuvenation Research. 2013;16(6):524–528. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

