Expanding the fragrance chemical space for virtual screening
- PMID: 24876890
- PMCID: PMC4037718
- DOI: 10.1186/1758-2946-6-27
Expanding the fragrance chemical space for virtual screening
Abstract
The properties of fragrance molecules in the public databases SuperScent and Flavornet were analyzed to define a "fragrance-like" (FL) property range (Heavy Atom Count ≤ 21, only C, H, O, S, (O + S) ≤ 3, Hydrogen Bond Donor ≤ 1) and the corresponding chemical space including FL molecules from PubChem (NIH repository of molecules), ChEMBL (bioactive molecules), ZINC (drug-like molecules), and GDB-13 (all possible organic molecules up to 13 atoms of C, N, O, S, Cl). The FL subsets of these databases were classified by MQN (Molecular Quantum Numbers, a set of 42 integer value descriptors of molecular structure) and formatted for fast MQN-similarity searching and interactive exploration of color-coded principal component maps in form of the FL-mapplet and FL-browser applications freely available at http://www.gdb.unibe.ch. MQN-similarity is shown to efficiently recover 15 different fragrance molecule families from the different FL subsets, demonstrating the relevance of the MQN-based tool to explore the fragrance chemical space.
Figures
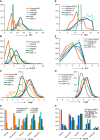
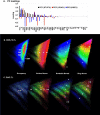
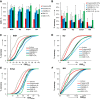
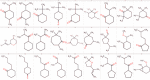
Similar articles
-
MQN-mapplet: visualization of chemical space with interactive maps of DrugBank, ChEMBL, PubChem, GDB-11, and GDB-13.J Chem Inf Model. 2013 Feb 25;53(2):509-18. doi: 10.1021/ci300513m. Epub 2013 Jan 22. J Chem Inf Model. 2013. PMID: 23297797
-
Visualization and virtual screening of the chemical universe database GDB-17.J Chem Inf Model. 2013 Jan 28;53(1):56-65. doi: 10.1021/ci300535x. Epub 2013 Jan 9. J Chem Inf Model. 2013. PMID: 23259841
-
Exploring the chemical space of known and unknown organic small molecules at www.gdb.unibe.ch.Chimia (Aarau). 2011;65(11):863-7. doi: 10.2533/chimia.2011.863. Chimia (Aarau). 2011. PMID: 22289373 Review.
-
Visualisation of the chemical space of fragments, lead-like and drug-like molecules in PubChem.J Comput Aided Mol Des. 2011 Jul;25(7):649-62. doi: 10.1007/s10822-011-9437-x. Epub 2011 May 27. J Comput Aided Mol Des. 2011. PMID: 21618008
-
Exploring chemical space for drug discovery using the chemical universe database.ACS Chem Neurosci. 2012 Sep 19;3(9):649-57. doi: 10.1021/cn3000422. Epub 2012 Apr 25. ACS Chem Neurosci. 2012. PMID: 23019491 Free PMC article. Review.
Cited by
-
Lost in chemical space? Maps to support organometallic catalysis.Chem Cent J. 2015 Jun 18;9:38. doi: 10.1186/s13065-015-0104-5. eCollection 2015. Chem Cent J. 2015. PMID: 26113874 Free PMC article.
-
Integrating ion mobility spectrometry into mass spectrometry-based exposome measurements: what can it add and how far can it go?Bioanalysis. 2017 Jan;9(1):81-98. doi: 10.4155/bio-2016-0244. Bioanalysis. 2017. PMID: 27921453 Free PMC article.
-
Identification of VOCs in essential oils extracted using ultrasound- and microwave-assisted methods from sweet cherry flower.Sci Rep. 2021 Jan 13;11(1):1167. doi: 10.1038/s41598-020-80891-0. Sci Rep. 2021. PMID: 33441964 Free PMC article.
-
Mapping odorant sensitivities reveals a sparse but structured representation of olfactory chemical space by sensory input to the mouse olfactory bulb.Elife. 2022 Jul 21;11:e80470. doi: 10.7554/eLife.80470. Elife. 2022. PMID: 35861321 Free PMC article.
-
BIGCHEM: Challenges and Opportunities for Big Data Analysis in Chemistry.Mol Inform. 2016 Dec;35(11-12):615-621. doi: 10.1002/minf.201600073. Epub 2016 Jul 28. Mol Inform. 2016. PMID: 27464907 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

