Hyperspectral and differential CARS microscopy for quantitative chemical imaging in human adipocytes
- PMID: 24877002
- PMCID: PMC4026891
- DOI: 10.1364/BOE.5.001378
Hyperspectral and differential CARS microscopy for quantitative chemical imaging in human adipocytes
Abstract
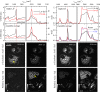
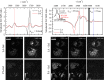
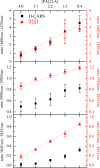
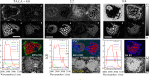
In this work, we demonstrate the applicability of coherent anti-Stokes Raman scattering (CARS) micro-spectroscopy for quantitative chemical imaging of saturated and unsaturated lipids in human stem-cell derived adipocytes. We compare dual-frequency/differential CARS (D-CARS), which enables rapid imaging and simple data analysis, with broadband hyperspectral CARS microscopy analyzed using an unsupervised phase-retrieval and factorization method recently developed by us for quantitative chemical image analysis. Measurements were taken in the vibrational fingerprint region (1200-2000/cm) and in the CH stretch region (2600-3300/cm) using a home-built CARS set-up which enables hyperspectral imaging with 10/cm resolution via spectral focussing from a single broadband 5 fs Ti:Sa laser source. Through a ratiometric analysis, both D-CARS and phase-retrieved hyperspectral CARS determine the concentration of unsaturated lipids with comparable accuracy in the fingerprint region, while in the CH stretch region D-CARS provides only a qualitative contrast owing to its non-linear behavior. When analyzing hyperspectral CARS images using the blind factorization into susceptibilities and concentrations of chemical components recently demonstrated by us, we are able to determine vol:vol concentrations of different lipid components and spatially resolve inhomogeneities in lipid composition with superior accuracy compared to state-of-the art ratiometric methods.
Keywords: (180.5655) Raman microscopy; (300.6230) Spectroscopy, coherent anti-Stokes Raman scattering.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
