The structural modeling of the interaction between levofloxacin and the Mycobacterium tuberculosis gyrase catalytic site sheds light on the mechanisms of fluoroquinolones resistant tuberculosis in Colombian clinical isolates
- PMID: 24877086
- PMCID: PMC4022255
- DOI: 10.1155/2014/367268
The structural modeling of the interaction between levofloxacin and the Mycobacterium tuberculosis gyrase catalytic site sheds light on the mechanisms of fluoroquinolones resistant tuberculosis in Colombian clinical isolates
Abstract
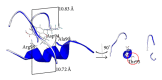
We compared the prevalence of levofloxacin (LVX) resistance with that of ofloxacin (OFX) and moxifloxacin (MFX) among multidrug resistant (MDR) MTB clinical isolates collected in Medellin, Colombia, between 2004 and 2009 and aimed at unraveling the underlying molecular mechanisms that explain the correlation between QRDR-A mutations and LVX resistance phenotype. We tested 104 MDR isolates for their susceptibility to OFX, MFX, and LVX. Resistance to OFX was encountered in 10 (9.6%) of the isolates among which 8 (7.7%) were also resistant to LVX and 6 (5.7%) to MFX. Four isolates resistant to the 3 FQ were harboring the Asp94Gly substitution, whilst 2 other isolates resistant to OFX and LVX presented the Ala90Val mutation. No mutations were found in the QRDR-B region. The molecular modeling of the interaction between LVX and the DNA-DNA gyrase complex indicates that the loss of an acetyl group in the Asp94Gly mutation removes the acid base interaction with LVX necessary for the quinolone activity. The Ala90Val mutation that substitutes a methyl for an isopropyl group induces a steric modification that blocks the LVX access to the gyrase catalytic site.
Figures


References
-
- WHO. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization; 2012.
-
- Nunn P. The global control of tuberculosis: what are the prospects? Scandinavian Journal of Infectious Diseases. 2001;33(5):329–332. - PubMed
-
- WHO. Framework for Effective Tuberculosis Control. Geneva, Switzerland: World Health Organization; 1994.
-
- Kochi A. Tuberculosis control- Is dots the health breakthrough of the 1990s? World Health Forum. 1997;18(3-4):225–232. - PubMed
-
- Keshavjee S, Farmer PE. Tuberculosis, drug resistance and the history of modern medicine. The New England Journal of Medicine. 2012;367:931–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

