The importance of the ionic product for water to understand the physiology of the acid-base balance in humans
- PMID: 24877130
- PMCID: PMC4022011
- DOI: 10.1155/2014/695281
The importance of the ionic product for water to understand the physiology of the acid-base balance in humans
Abstract
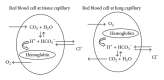
Human plasma is an aqueous solution that has to abide by chemical rules such as the principle of electrical neutrality and the constancy of the ionic product for water. These rules define the acid-base balance in the human body. According to the electroneutrality principle, plasma has to be electrically neutral and the sum of its cations equals the sum of its anions. In addition, the ionic product for water has to be constant. Therefore, the plasma concentration of hydrogen ions depends on the plasma ionic composition. Variations in the concentration of plasma ions that alter the relative proportion of anions and cations predictably lead to a change in the plasma concentration of hydrogen ions by driving adaptive adjustments in water ionization that allow plasma electroneutrality while maintaining constant the ionic product for water. The accumulation of plasma anions out of proportion of cations induces an electrical imbalance compensated by a fall of hydroxide ions that brings about a rise in hydrogen ions (acidosis). By contrast, the deficiency of chloride relative to sodium generates plasma alkalosis by increasing hydroxide ions. The adjustment of plasma bicarbonate concentration to these changes is an important compensatory mechanism that protects plasma pH from severe deviations.
Similar articles
-
[Clinical evaluation of acid-base status: Henderson-Hasselbalch, or Stewart-Fencl approach?].Cas Lek Cesk. 2016 Winter;155(7):365-369. Cas Lek Cesk. 2016. PMID: 27990831 Review. Czech.
-
Determinants of blood pH in health and disease.Crit Care. 2000;4(1):6-14. doi: 10.1186/cc644. Epub 2000 Jan 24. Crit Care. 2000. PMID: 11094491 Free PMC article. Review.
-
Strong ions and charge-balance.Scand J Clin Lab Invest. 2023 Apr;83(2):111-118. doi: 10.1080/00365513.2023.2180658. Epub 2023 Feb 22. Scand J Clin Lab Invest. 2023. PMID: 36811448
-
Introduction to an alternate view of acid/base balance: the strong ion difference or Stewart approach.Aust Crit Care. 2002 Feb;15(1):14-20. doi: 10.1016/s1036-7314(02)80039-x. Aust Crit Care. 2002. PMID: 11979985
-
Acid-base balance: origin of plasma [H+] during exercise.Can J Appl Physiol. 1995 Sep;20(3):341-56. doi: 10.1139/h95-027. Can J Appl Physiol. 1995. PMID: 8541797 Review.
Cited by
-
A Review on Recent Development of Phenothiazine-Based Chromogenic and Fluorogenic Sensors for the Detection of Cations, Anions, and Neutral Analytes.Top Curr Chem (Cham). 2024 Sep 5;382(3):29. doi: 10.1007/s41061-024-00474-9. Top Curr Chem (Cham). 2024. PMID: 39237745 Review.
-
Effects and interaction of dietary electrolyte balance and citric acid on the intestinal function of weaned piglets.J Anim Sci. 2020 May 1;98(5):skaa106. doi: 10.1093/jas/skaa106. J Anim Sci. 2020. PMID: 32253427 Free PMC article.
-
Label-Free Protein Detection by Micro-Acoustic Biosensor Coupled with Electrical Field Sorting. Theoretical Study in Urine Models.Sensors (Basel). 2021 Apr 6;21(7):2555. doi: 10.3390/s21072555. Sensors (Basel). 2021. PMID: 33917374 Free PMC article.
-
Fluid dynamics of life: exploring the physiology and importance of water in the critical illness.Front Med (Lausanne). 2024 Apr 30;11:1368502. doi: 10.3389/fmed.2024.1368502. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38745736 Free PMC article. Review.
-
Possible toxicity of chronic carbon dioxide exposure associated with face mask use, particularly in pregnant women, children and adolescents - A scoping review.Heliyon. 2023 Apr;9(4):e14117. doi: 10.1016/j.heliyon.2023.e14117. Epub 2023 Mar 3. Heliyon. 2023. PMID: 37057051 Free PMC article.
References
-
- Stewart PA. Modern quantitative acid-base chemistry. Canadian Journal of Physiology and Pharmacology. 1983;61(12):1444–1461. - PubMed
-
- Story DA, Morimatsu H, Bellomo R. Strong ions, weak acids and base excess: a simplified Fencl-Stewart approach to clinical acid-base disorders. British Journal of Anaesthesia. 2004;92(1):54–60. - PubMed
-
- Nagaoka D, Nassar Junior AP, Maciel AT, et al. The use of sodium-chloride difference and chloride-sodium ratio as strong ion difference surrogates in the evaluation of metabolic acidosis in critically ill patients. Journal of Critical Care. 2010;25(3):525–531. - PubMed
-
- McNamara J, Worthley LI. Acid-base balance: part I. Physiology. Critical Care and Resuscitation. 2001;3(3):181–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical