Maternal thyroid hormones are essential for neural development in zebrafish
- PMID: 24877564
- PMCID: PMC5414824
- DOI: 10.1210/me.2014-1032
Maternal thyroid hormones are essential for neural development in zebrafish
Abstract
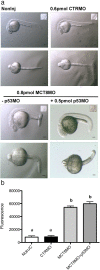
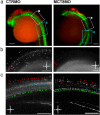
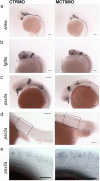
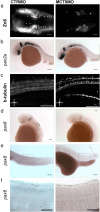
Teleost eggs contain an abundant store of maternal thyroid hormones (THs), and early in zebrafish embryonic development, all the genes necessary for TH signaling are expressed. Nonetheless the function of THs in embryonic development remains elusive. To test the hypothesis that THs are fundamental for zebrafish embryonic development, an monocarboxilic transporter 8 (Mct8) knockdown strategy was deployed to prevent maternal TH uptake. Absence of maternal THs did not affect early specification of the neural epithelia but profoundly modified later dorsal specification of the brain and spinal cord as well as specific neuron differentiation. Maternal THs acted upstream of pax2a, pax7, and pax8 genes but downstream of shha and fgf8a signaling. The lack of inhibitory spinal cord interneurons and increased motoneurons in the mct8 morphants is consistent with their stiff axial body and impaired mobility. The mct8 mutations are associated with X-linked mental retardation in humans, and the cellular and molecular consequences of MCT8 knockdown during embryonic development in zebrafish provides new insight into the potential role of THs in this condition.
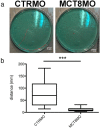
Figures



Similar articles
-
From zebrafish to human: A comparative approach to elucidate the role of the thyroid hormone transporter MCT8 during brain development.Gen Comp Endocrinol. 2018 Sep 1;265:219-229. doi: 10.1016/j.ygcen.2017.11.023. Epub 2017 Nov 26. Gen Comp Endocrinol. 2018. PMID: 29183795 Review.
-
Deficiency of the Thyroid Hormone Transporter Monocarboxylate Transporter 8 in Neural Progenitors Impairs Cellular Processes Crucial for Early Corticogenesis.J Neurosci. 2017 Nov 29;37(48):11616-11631. doi: 10.1523/JNEUROSCI.1917-17.2017. Epub 2017 Nov 6. J Neurosci. 2017. PMID: 29109240 Free PMC article.
-
Knockdown of the thyroid hormone transporter MCT8 in chicken retinal precursor cells hampers early retinal development and results in a shift towards more UV/blue cones at the expense of green/red cones.Exp Eye Res. 2019 Jan;178:135-147. doi: 10.1016/j.exer.2018.09.018. Epub 2018 Sep 28. Exp Eye Res. 2019. PMID: 30273578
-
Zebrafish - An emerging model to explore thyroid hormone transporters and psychomotor retardation.Mol Cell Endocrinol. 2017 Dec 25;459:53-58. doi: 10.1016/j.mce.2017.03.004. Epub 2017 Mar 6. Mol Cell Endocrinol. 2017. PMID: 28274736 Review.
-
Maternal thyroid hormone is required to develop the hindbrain vasculature in zebrafish.Commun Biol. 2025 Jul 1;8(1):960. doi: 10.1038/s42003-025-08404-1. Commun Biol. 2025. PMID: 40593336 Free PMC article.
Cited by
-
Differential expression of olfactory genes in Atlantic salmon (Salmo salar) during the parr-smolt transformation.Ecol Evol. 2019 Nov 28;9(24):14085-14100. doi: 10.1002/ece3.5845. eCollection 2019 Dec. Ecol Evol. 2019. PMID: 31938505 Free PMC article.
-
In a zebrafish biomedical model of human Allan-Herndon-Dudley syndrome impaired MTH signaling leads to decreased neural cell diversity.Front Endocrinol (Lausanne). 2023 May 4;14:1157685. doi: 10.3389/fendo.2023.1157685. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37214246 Free PMC article.
-
Genetic and Neurological Deficiencies in the Visual System of mct8 Mutant Zebrafish.Int J Mol Sci. 2022 Feb 23;23(5):2464. doi: 10.3390/ijms23052464. Int J Mol Sci. 2022. PMID: 35269606 Free PMC article.
-
The transcriptome of metamorphosing flatfish.BMC Genomics. 2016 May 27;17:413. doi: 10.1186/s12864-016-2699-x. BMC Genomics. 2016. PMID: 27233904 Free PMC article.
-
Mob4 is required for neurodevelopment in zebrafish.MicroPubl Biol. 2023 Feb 24;2023:10.17912/micropub.biology.000762. doi: 10.17912/micropub.biology.000762. eCollection 2023. MicroPubl Biol. 2023. PMID: 36915897 Free PMC article.
References
-
- Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice. Endocrinology. 2006;147(9):4036–4043. - PubMed
-
- Horn S, Heuer H. Thyroid hormone action during brain development: More questions than answers. Mol Cell Endocrinol. 2010;315(1–2):19–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

