Massive formation of square array junctions dramatically alters cell shape but does not cause lens opacity in the cav1-KO mice
- PMID: 24877741
- PMCID: PMC4123542
- DOI: 10.1016/j.exer.2014.05.014
Massive formation of square array junctions dramatically alters cell shape but does not cause lens opacity in the cav1-KO mice
Abstract
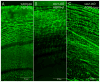
The wavy square array junctions are composed of truncated aquaporin-0 (AQP0) proteins typically distributed in the deep cortical and nuclear fibers in wild-type lenses. These junctions may help maintain the narrowed extracellular spaces between fiber cells to minimize light scattering. Herein, we investigate the impact of the cell shape changes, due to abnormal formation of extensive square array junctions, on the lens opacification in the caveolin-1 knockout mice. The cav1-KO and wild-type mice at age 1-22 months were used. By light microscopy examinations, cav1-KO lenses at age 1-18 months were transparent in both cortical and nuclear regions, whereas some lenses older than 18 months old exhibited nuclear cataracts. Scanning EM consistently observed the massive formation of ridge-and-valley membrane surfaces in young fibers at approximately 150 μm deep in all cav1-KO lenses studied. In contrast, the typical ridge-and-valleys were only seen in mature fibers deeper than 400 μm in wild-type lenses. The resulting extensive ridge-and-valleys dramatically altered the overall cell shape in cav1-KO lenses. Remarkably, despite dramatic shape changes, these deformed fiber cells remained intact and made close contact with their neighboring cells. By freeze-fracture TEM, ridge-and-valleys exhibited the typical orthogonal arrangement of 6.6 nm square array intramembrane particles and displayed the narrowed extracellular spaces. Immunofluorescence analysis showed that AQP0 C-terminus labeling was significantly decreased in outer cortical fibers in cav1-KO lenses. However, freeze-fracture immunogold labeling showed that the AQP0 C-terminus antibody was sparsely distributed on the wavy square array junctions, suggesting that the cleavage of AQP0 C-termini might not yet be complete. The cav1-KO lenses with nuclear cataracts showed complete cellular breakdown and large globule formation in the lens nucleus. This study suggests that despite dramatic cell shape changes, the massive formation of wavy square array junctions in intact fibers may provide additional adhesive support for maintaining the narrowed extracellular spaces that are crucial for the transparency of cav1-KO lenses.
Keywords: AQP0; caveolin-1 knockout; fiber cell membrane; lens; mouse; square array junctions.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures







References
-
- Alcala J, Lieska N, Maisel H. Protein composition of bovine lens cortical fiber cell membranes. Exp Eye Res. 1975;21:581–95. - PubMed
-
- Alcala J, Putt D, Maisel H. Limited proteolysis of gap junction protein is intrinsic in mammalian lens fiber-cell plasma membranes. Biochem Biophys Res Commun. 1987;147:846–53. - PubMed
-
- Alcala J, Valentine J, Maisel H. Human lens fiber cell plasma membranes. I. Isolation, polypeptide composition and changes associated with ageing. Exp Eye Res. 1980;30:659–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

