Ranolazine inhibition of hERG potassium channels: drug-pore interactions and reduced potency against inactivation mutants
- PMID: 24877995
- PMCID: PMC4121676
- DOI: 10.1016/j.yjmcc.2014.05.013
Ranolazine inhibition of hERG potassium channels: drug-pore interactions and reduced potency against inactivation mutants
Abstract
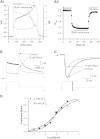
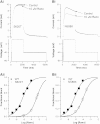
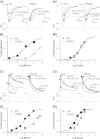
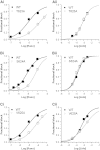
The antianginal drug ranolazine, which combines inhibitory actions on rapid and sustained sodium currents with inhibition of the hERG/IKr potassium channel, shows promise as an antiarrhythmic agent. This study investigated the structural basis of hERG block by ranolazine, with lidocaine used as a low potency, structurally similar comparator. Recordings of hERG current (IhERG) were made from cell lines expressing wild-type (WT) or mutant hERG channels. Docking simulations were performed using homology models built on MthK and KvAP templates. In conventional voltage clamp, ranolazine inhibited IhERG with an IC50 of 8.03μM; peak IhERG during ventricular action potential clamp was inhibited ~62% at 10μM. The IC50 values for ranolazine inhibition of the S620T inactivation deficient and N588K attenuated inactivation mutants were respectively ~73-fold and ~15-fold that for WT IhERG. Mutations near the bottom of the selectivity filter (V625A, S624A, T623A) exhibited IC50s between ~8 and 19-fold that for WT IhERG, whilst the Y652A and F656A S6 mutations had IC50s ~22-fold and 53-fold WT controls. Low potency lidocaine was comparatively insensitive to both pore helix and S6 mutations, but was sensitive to direction of K(+) flux and particularly to loss of inactivation, with an IC50 for S620T-hERG ~49-fold that for WT IhERG. Docking simulations indicated that the larger size of ranolazine gives it potential for a greater range of interactions with hERG pore side chains compared to lidocaine, in particular enabling interaction of its two aromatic groups with side chains of both Y652 and F656. The N588K mutation is responsible for the SQT1 variant of short QT syndrome and our data suggest that ranolazine is unlikely to be effective against IKr/hERG in SQT1 patients.
Keywords: Antiarrhythmic; Docking; Lidocaine; QT interval; Ranolazine; hERG.
Copyright © 2014. Published by Elsevier Ltd.
Figures
Similar articles
-
Interactions between amiodarone and the hERG potassium channel pore determined with mutagenesis and in silico docking.Biochem Pharmacol. 2016 Aug 1;113:24-35. doi: 10.1016/j.bcp.2016.05.013. Epub 2016 May 30. Biochem Pharmacol. 2016. PMID: 27256139 Free PMC article.
-
Molecular basis of hERG potassium channel blockade by the class Ic antiarrhythmic flecainide.J Mol Cell Cardiol. 2015 Sep;86:42-53. doi: 10.1016/j.yjmcc.2015.06.021. Epub 2015 Jul 6. J Mol Cell Cardiol. 2015. PMID: 26159617 Free PMC article.
-
Molecular determinants of hERG potassium channel inhibition by disopyramide.J Mol Cell Cardiol. 2012 Jan;52(1):185-95. doi: 10.1016/j.yjmcc.2011.09.021. Epub 2011 Sep 29. J Mol Cell Cardiol. 2012. PMID: 21989164
-
The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications.Curr Pharm Des. 2006;12(18):2271-83. doi: 10.2174/138161206777585102. Curr Pharm Des. 2006. PMID: 16787254 Review.
-
Electrophysiologic basis for the antiarrhythmic actions of ranolazine.Heart Rhythm. 2011 Aug;8(8):1281-90. doi: 10.1016/j.hrthm.2011.03.045. Epub 2011 Mar 21. Heart Rhythm. 2011. PMID: 21421082 Free PMC article. Review.
Cited by
-
Suppression of the hERG potassium channel response to premature stimulation by reduction in extracellular potassium concentration.Physiol Rep. 2014 Oct 15;2(10):e12165. doi: 10.14814/phy2.12165. Print 2014 Oct 1. Physiol Rep. 2014. PMID: 25318749 Free PMC article.
-
A structure-based computational workflow to predict liability and binding modes of small molecules to hERG.Sci Rep. 2020 Oct 1;10(1):16262. doi: 10.1038/s41598-020-72889-5. Sci Rep. 2020. PMID: 33004839 Free PMC article.
-
Calculation of absolute binding free energies between the hERG channel and structurally diverse drugs.Sci Rep. 2019 Nov 12;9(1):16586. doi: 10.1038/s41598-019-53120-6. Sci Rep. 2019. PMID: 31719645 Free PMC article.
-
Computational Modeling of Electrophysiology and Pharmacotherapy of Atrial Fibrillation: Recent Advances and Future Challenges.Front Physiol. 2018 Sep 4;9:1221. doi: 10.3389/fphys.2018.01221. eCollection 2018. Front Physiol. 2018. PMID: 30233399 Free PMC article. Review.
-
Delayed Ventricular Repolarization and Sodium Channel Current Modification in a Mouse Model of Rett Syndrome.Int J Mol Sci. 2022 May 20;23(10):5735. doi: 10.3390/ijms23105735. Int J Mol Sci. 2022. PMID: 35628543 Free PMC article.
References
-
- Nattel S. Class III drugs: amiodarone, bretylium, ibutilide and sotalol. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology; from cell to bedside. 3rd ed. 1999. pp. 921–932.
-
- Hondeghem L.M., Snyders D.J. Class III antiarrhythmic agents have a lot of potential but a long way to go. Circulation. 1990;81:686–690. - PubMed
-
- Sanguinetti M.C., Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. March 23 2006;440:463–469. - PubMed
-
- Hancox J.C., McPate M.J., El Harchi A., Zhang Y.H. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther. 2008;119:118–132. - PubMed
-
- Lees-Miller J.P., Duan Y., Teng G.Q., Duff H.J. Molecular determinant of high affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol. 2000;57:367–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

