The deubiquitinase activity of A20 is dispensable for NF-κB signaling
- PMID: 24878851
- PMCID: PMC4196981
- DOI: 10.15252/embr.201338305
The deubiquitinase activity of A20 is dispensable for NF-κB signaling
Abstract
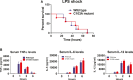
A20 has been suggested to limit NF-κB activation by removing regulatory ubiquitin chains from ubiquitinated substrates. A20 is a ubiquitin-editing enzyme that removes K63-linked ubiquitin chains from adaptor proteins, such as RIP1, and then conjugates them to K48-linked polyubiquitin chains to trigger proteasomal degradation. To determine the role of the deubiquitinase function of A20 in downregulating NF-κB signaling, we have generated a knock-in mouse that lacks the deubiquitinase function of A20 (A20-OTU mice). These mice are normal and have no signs of inflammation, have normal proportions of B, T, dendritic, and myeloid cells, respond normally to LPS and TNF, and undergo normal NF-κB activation. Our results thus indicate that the deubiquitinase activity of A20 is dispensable for normal NF-κB signaling.
Keywords: A20; K63‐linked ubiquitination; NF‐κB; deubiquitinase; signal transduction.
© 2014 The Authors.
Figures




Comment in
-
A20: attractive without showing cleavage.EMBO Rep. 2014 Jul;15(7):734-5. doi: 10.15252/embr.201439014. Epub 2014 May 30. EMBO Rep. 2014. PMID: 24878850 Free PMC article.
References
-
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. - PubMed
-
- Opipari AW, Jr, Hu HM, Yabkowitz R, Dixit VM. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. - PubMed
-
- Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996;156:1166–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

