Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells
- PMID: 24878890
- PMCID: PMC4221287
- DOI: 10.1007/s00262-014-1561-8
Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells
Abstract
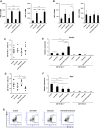
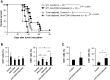
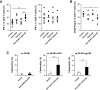
Malignant gliomas are heavily infiltrated by immature myeloid cells that mediate immunosuppression. Agonistic CD40 monoclonal antibody (mAb) has been shown to activate myeloid cells and promote antitumor immunity. Our previous study has also demonstrated blockade of cyclooxygenase-2 (COX-2) reduces immunosuppressive myeloid cells, thereby suppressing glioma development in mice. We therefore hypothesized that a combinatory strategy to modulate myeloid cells via two distinct pathways, i.e., CD40/CD40L stimulation and COX-2 blockade, would enhance anti-glioma immunity. We used three different mouse glioma models to evaluate therapeutic effects and underlying mechanisms of a combination regimen with an agonist CD40 mAb and the COX-2 inhibitor celecoxib. Treatment of glioma-bearing mice with the combination therapy significantly prolonged survival compared with either anti-CD40 mAb or celecoxib alone. The combination regimen promoted maturation of CD11b(+) cells in both spleen and brain, and enhanced Cxcl10 while suppressing Arg1 in CD11b(+)Gr-1(+) cells in the brain. Anti-glioma activity of the combination regimen was T-cell dependent because depletion of CD4(+) and CD8(+) cells in vivo abrogated the anti-glioma effects. Furthermore, the combination therapy significantly increased the frequency of CD8(+) T-cells, enhanced IFN-γ-production and reduced CD4(+)CD25(+)Foxp3(+) T regulatory cells in the brain, and induced tumor-antigen-specific T-cell responses in lymph nodes. Our findings suggest that the combination therapy of anti-CD40 mAb with celecoxib enhances anti-glioma activities via promotion of type-1 immunity both in myeloid cells and T-cells.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

