A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP
- PMID: 24878962
- PMCID: PMC4139233
- DOI: 10.1074/jbc.M114.572164
A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP
Abstract
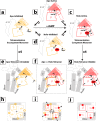
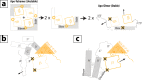
Hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels control neuronal and cardiac electrical rhythmicity. There are four homologous isoforms (HCN1-4) sharing a common multidomain architecture that includes an N-terminal transmembrane tetrameric ion channel followed by a cytoplasmic "C-linker," which connects a more distal cAMP-binding domain (CBD) to the inner pore. Channel opening is primarily stimulated by transmembrane elements that sense membrane hyperpolarization, although cAMP reduces the voltage required for HCN activation by promoting tetramerization of the intracellular C-linker, which in turn relieves auto-inhibition of the inner pore gate. Although binding of cAMP has been proposed to relieve auto-inhibition by affecting the structure of the C-linker and CBD, the nature and extent of these cAMP-dependent changes remain limitedly explored. Here, we used NMR to probe the changes caused by the binding of cAMP and of cCMP, a partial agonist, to the apo-CBD of HCN4. Our data indicate that the CBD exists in a dynamic two-state equilibrium, whose position as gauged by NMR chemical shifts correlates with the V½ voltage measured through electrophysiology. In the absence of cAMP, the most populated CBD state leads to steric clashes with the activated or "tetrameric" C-linker, which becomes energetically unfavored. The steric clashes of the apo tetramer are eliminated either by cAMP binding, which selects for a CBD state devoid of steric clashes with the tetrameric C-linker and facilitates channel opening, or by a transition of apo-HCN to monomers or dimer of dimers, in which the C-linker becomes less structured, and channel opening is not facilitated.
Keywords: Allosteric Regulation; Allostery; Cyclic AMP (cAMP); HCN; Intrinsically Disordered Proteins (IDPs); Nuclear Magnetic Resonance (NMR); Protein Conformation; Signaling; cAMP-binding Domain (CBD); cCMP.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Zagotta W. N., Olivier N. B., Black K. D., Young E. C., Olson R., Gouaux E. (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 - PubMed
-
- Robinson R. B., Siegelbaum S. A. (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65, 453–480 - PubMed
-
- Trudeau M. C., Zagotta W. N. (2003) Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels. J. Biol. Chem. 278, 18705–18708 - PubMed
-
- Kaupp U. B., Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824 - PubMed
-
- DiFrancesco D., Tortora P. (1991) Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351, 145–147 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

