Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae
- PMID: 24879123
- PMCID: PMC4187654
- DOI: 10.1128/EC.00080-14
Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae
Abstract
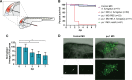
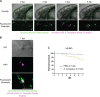
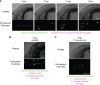
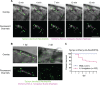
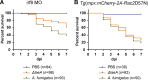
Aspergillus fumigatus is the most common filamentous fungal pathogen of immunocompromised hosts, resulting in invasive aspergillosis (IA) and high mortality rates. Innate immunity is known to be the predominant host defense against A. fumigatus; however, innate phagocyte responses to A. fumigatus in an intact host and their contributions to host survival remain unclear. Here, we describe a larval zebrafish A. fumigatus infection model amenable to real-time imaging of host-fungal interactions in live animals. Following infection with A. fumigatus, innate phagocyte populations exhibit clear preferences for different fungal morphologies: macrophages rapidly phagocytose conidia and form aggregates around hyphae, while the neutrophil response is dependent upon the presence of hyphae. Depletion of macrophages rendered host larvae susceptible to invasive disease. Moreover, a zebrafish model of human leukocyte adhesion deficiency with impaired neutrophil function also resulted in invasive disease and impaired host survival. In contrast, macrophage-deficient but not neutrophil-deficient larvae exhibited attenuated disease following challenge with a less virulent (ΔlaeA) strain of A. fumigatus, which has defects in secondary metabolite production. Taking these results together, we have established a new vertebrate model for studying innate immune responses to A. fumigatus that reveals distinct roles for neutrophils and macrophages in mediating host defense against IA.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Real-time visualization of immune cell clearance of Aspergillus fumigatus spores and hyphae.Fungal Genet Biol. 2017 Aug;105:52-54. doi: 10.1016/j.fgb.2017.05.005. Epub 2017 May 27. Fungal Genet Biol. 2017. PMID: 28559109 Free PMC article.
-
Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects.J Immunol. 2016 Feb 1;196(3):1272-83. doi: 10.4049/jimmunol.1501811. Epub 2015 Dec 30. J Immunol. 2016. PMID: 26718340
-
Interaction of phagocytes with filamentous fungi.Curr Opin Microbiol. 2010 Aug;13(4):409-15. doi: 10.1016/j.mib.2010.04.009. Epub 2010 Jun 2. Curr Opin Microbiol. 2010. PMID: 20627805 Review.
-
Larval zebrafish burn wound infection model reveals conserved innate immune responses against diverse pathogenic fungi.mBio. 2025 May 14;16(5):e0348024. doi: 10.1128/mbio.03480-24. Epub 2025 Apr 8. mBio. 2025. PMID: 40197062 Free PMC article.
-
Phagocyte responses towards Aspergillus fumigatus.Int J Med Microbiol. 2011 Jun;301(5):436-44. doi: 10.1016/j.ijmm.2011.04.012. Epub 2011 May 14. Int J Med Microbiol. 2011. PMID: 21571589 Review.
Cited by
-
The Zebrafish as a Model Host for Invasive Fungal Infections.J Fungi (Basel). 2018 Dec 13;4(4):136. doi: 10.3390/jof4040136. J Fungi (Basel). 2018. PMID: 30551557 Free PMC article. Review.
-
An Overlooked and Underrated Endemic Mycosis-Talaromycosis and the Pathogenic Fungus Talaromyces marneffei.Clin Microbiol Rev. 2023 Mar 23;36(1):e0005122. doi: 10.1128/cmr.00051-22. Epub 2023 Jan 17. Clin Microbiol Rev. 2023. PMID: 36648228 Free PMC article. Review.
-
Real-time imaging of inflammation and its resolution: It's apparent because it's transparent.Immunol Rev. 2022 Mar;306(1):258-270. doi: 10.1111/imr.13061. Epub 2022 Jan 12. Immunol Rev. 2022. PMID: 35023170 Free PMC article. Review.
-
Functional Characterization of Clinical Isolates of the Opportunistic Fungal Pathogen Aspergillus nidulans.mSphere. 2020 Apr 8;5(2):e00153-20. doi: 10.1128/mSphere.00153-20. mSphere. 2020. PMID: 32269156 Free PMC article.
-
Zebrafish (Danio rerio) as a Model System to Investigate the Role of the Innate Immune Response in Human Infectious Diseases.Int J Mol Sci. 2024 Nov 8;25(22):12008. doi: 10.3390/ijms252212008. Int J Mol Sci. 2024. PMID: 39596075 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

