Identification of a novel MTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells
- PMID: 24879147
- PMCID: PMC4091179
- DOI: 10.4161/auto.28363
Identification of a novel MTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells
Abstract
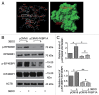
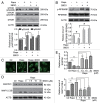
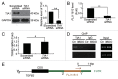
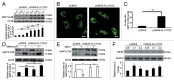
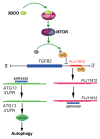
MTOR, a central regulator of autophagy, is involved in cancer and cardiovascular and neurological diseases. Modulating the MTOR signaling balance could be of great significance for numerous diseases. No chemical activators of MTOR have been found, and the urgent challenge is to find novel MTOR downstream components. In previous studies, we found a chemical small molecule, 3-benzyl-5-((2-nitrophenoxy) methyl)-dihydrofuran-2(3H)-one (3BDO), that inhibited autophagy in human umbilical vein endothelial cells (HUVECs) and neuronal cells. Here, we found that 3BDO activated MTOR by targeting FKBP1A (FK506-binding protein 1A, 12 kDa). We next used 3BDO to detect novel factors downstream of the MTOR signaling pathway. Activation of MTOR by 3BDO increased the phosphorylation of TIA1 (TIA1 cytotoxic granule-associated RNA binding protein/T-cell-restricted intracellular antigen-1). Finally, we used gene microarray, RNA interference, RNA-ChIP assay, bioinformatics, luciferase reporter assay, and other assays and found that 3BDO greatly decreased the level of a long noncoding RNA (lncRNA) derived from the 3' untranslated region (3'UTR) of TGFB2, known as FLJ11812. TIA1 was responsible for processing FLJ11812. Further experiments results showed that FLJ11812 could bind with MIR4459 targeting ATG13 (autophagy-related 13), and ATG13 protein level was decreased along with 3BDO-decreased FLJ11812 level. Here, we provide a new activator of MTOR, and our findings highlight the role of the lncRNA in autophagy.
Keywords: ATG13; MIR4459; MTOR activator; TIA1; autophagy; lncRNA.
Figures




Similar articles
-
A 3'UTR-associated RNA, FLJ11812 maintains stemness of human embryonic stem cells by targeting miR-4459.Stem Cells Dev. 2015 May 1;24(9):1133-40. doi: 10.1089/scd.2014.0353. Epub 2015 Jan 13. Stem Cells Dev. 2015. PMID: 25437332 Free PMC article.
-
A new microRNA signal pathway regulated by long noncoding RNA TGFB2-OT1 in autophagy and inflammation of vascular endothelial cells.Autophagy. 2015;11(12):2172-83. doi: 10.1080/15548627.2015.1106663. Autophagy. 2015. PMID: 26565952 Free PMC article.
-
An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E⁻/⁻ mice.Sci Rep. 2014 Jul 1;4:5519. doi: 10.1038/srep05519. Sci Rep. 2014. PMID: 24980430 Free PMC article.
-
The Multifunctional Faces of T-Cell Intracellular Antigen 1 in Health and Disease.Int J Mol Sci. 2022 Jan 26;23(3):1400. doi: 10.3390/ijms23031400. Int J Mol Sci. 2022. PMID: 35163320 Free PMC article. Review.
-
Unraveling the Roles of Protein Kinases in Autophagy: An Update on Small-Molecule Compounds for Targeted Therapy.J Med Chem. 2022 Apr 28;65(8):5870-5885. doi: 10.1021/acs.jmedchem.1c02053. Epub 2022 Apr 7. J Med Chem. 2022. PMID: 35390258 Review.
Cited by
-
An autophagy-related long non-coding RNA signature in tongue squamous cell carcinoma.BMC Oral Health. 2023 Feb 22;23(1):120. doi: 10.1186/s12903-023-02806-5. BMC Oral Health. 2023. PMID: 36814212 Free PMC article.
-
RNA helicase DHX15 exemplifies a unique dependency in acute leukemia.Haematologica. 2023 Aug 1;108(8):2029-2043. doi: 10.3324/haematol.2022.282066. Haematologica. 2023. PMID: 36861414 Free PMC article.
-
New Mammalian Target of Rapamycin (mTOR) Modulators Derived from Natural Product Databases and Marine Extracts by Using Molecular Docking Techniques.Mar Drugs. 2018 Oct 15;16(10):385. doi: 10.3390/md16100385. Mar Drugs. 2018. PMID: 30326670 Free PMC article.
-
Long Non-coding RNA H19 Induces Cerebral Ischemia Reperfusion Injury via Activation of Autophagy.Aging Dis. 2017 Feb 1;8(1):71-84. doi: 10.14336/AD.2016.0530. eCollection 2017 Feb. Aging Dis. 2017. PMID: 28203482 Free PMC article.
-
A New Long Noncoding RNA ALB Regulates Autophagy by Enhancing the Transformation of LC3BI to LC3BII during Human Lens Development.Mol Ther Nucleic Acids. 2017 Dec 15;9:207-217. doi: 10.1016/j.omtn.2017.09.011. Epub 2017 Oct 5. Mol Ther Nucleic Acids. 2017. PMID: 29246299 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
