Phosphorylation of NBR1 by GSK3 modulates protein aggregation
- PMID: 24879152
- PMCID: PMC4091167
- DOI: 10.4161/auto.28479
Phosphorylation of NBR1 by GSK3 modulates protein aggregation
Abstract
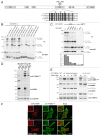
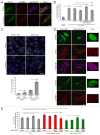
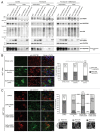
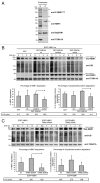
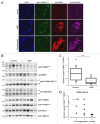
The autophagy receptor NBR1 (neighbor of BRCA1 gene 1) binds UB/ubiquitin and the autophagosome-conjugated MAP1LC3/LC3 (microtubule-associated protein 1 light chain 3) proteins, thereby ensuring ubiquitinated protein degradation. Numerous neurodegenerative and neuromuscular diseases are associated with inappropriate aggregation of ubiquitinated proteins and GSK3 (glycogen synthase kinase 3) activity is involved in several of these proteinopathies. Here we show that NBR1 is a substrate of GSK3. NBR1 phosphorylation by GSK3 at Thr586 prevents the aggregation of ubiquitinated proteins and their selective autophagic degradation. Indeed, NBR1 phosphorylation decreases protein aggregation induced by puromycin or by the DES/desmin N342D mutant found in desminopathy patients and stabilizes ubiquitinated proteins. Importantly, decrease of protein aggregates is due to an inhibition of their formation and not to their autophagic degradation as confirmed by data on Atg7 knockout mice. The relevance of NBR1 phosphorylation in human pathology was investigated. Analysis of muscle biopsies of sporadic inclusion body myositis (sIBM) patients revealed a strong decrease of NBR1 phosphorylation in muscles of sIBM patients that directly correlated with the severity of protein aggregation. We propose that phosphorylation of NBR1 by GSK3 modulates the formation of protein aggregates and that this regulation mechanism is defective in a human muscle proteinopathy.
Keywords: GSK3; NBR1; autophagy; sIBM; skeletal muscle; sporadic inclusion body myositis; ubiquitinated protein aggregates.
Figures
References
-
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
