Cyclin A2, a novel regulator of EMT
- PMID: 24879294
- PMCID: PMC11113891
- DOI: 10.1007/s00018-014-1654-8
Cyclin A2, a novel regulator of EMT
Abstract
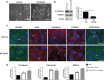
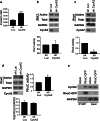
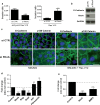
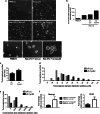
Our previous work showed that Cyclin A2 deficiency promotes cell invasion in fibroblasts. Given that the majority of cancers emerge from epithelia, we explored novel functions for Cyclin A2 by depleting it in normal mammary epithelial cells. This caused an epithelial to mesenchymal transition (EMT) associated with loss of cell-to-cell contacts, decreased E-Cadherin expression and increased invasive properties characterized by a reciprocal regulation of RhoA and RhoC activities, where RhoA-decreased activity drove cell invasiveness and E-Cadherin delocalization, and RhoC-increased activity only supported cell motility. Phenotypes induced by Cyclin A2 deficiency were exacerbated upon oncogenic activated-Ras expression, which led to an increased expression of EMT-related transcriptional factors. Moreover, Cyclin A2-depleted cells exhibited stem cell-like properties and increased invasion in an in vivo avian embryo model. Our work supports a model where Cyclin A2 downregulation facilitates cancer cell EMT and metastatic dissemination.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients.Oncotarget. 2014 Nov 15;5(21):10803-15. doi: 10.18632/oncotarget.2506. Oncotarget. 2014. PMID: 25301732 Free PMC article.
-
A novel function for Cyclin A2: control of cell invasion via RhoA signaling.J Cell Biol. 2012 Jan 9;196(1):147-62. doi: 10.1083/jcb.201102085. J Cell Biol. 2012. PMID: 22232705 Free PMC article.
-
Cyclin A2, Rho GTPases and EMT.Small GTPases. 2012 Oct-Dec;3(4):225-8. doi: 10.4161/sgtp.20791. Epub 2012 Jun 27. Small GTPases. 2012. PMID: 22735340 Free PMC article.
-
Inducible expression of Oct-3/4 reveals synergy with Klf4 in targeting Cyclin A2 to enhance proliferation during early reprogramming.Biochem Biophys Res Commun. 2022 Jan 8;587:29-35. doi: 10.1016/j.bbrc.2021.11.058. Epub 2021 Nov 19. Biochem Biophys Res Commun. 2022. PMID: 34864392
-
Cyclin A2: At the crossroads of cell cycle and cell invasion.World J Biol Chem. 2015 Nov 26;6(4):346-50. doi: 10.4331/wjbc.v6.i4.346. World J Biol Chem. 2015. PMID: 26629317 Free PMC article. Review.
Cited by
-
Regulating the CCNB1 gene can affect cell proliferation and apoptosis in pituitary adenomas and activate epithelial-to-mesenchymal transition.Oncol Lett. 2019 Nov;18(5):4651-4658. doi: 10.3892/ol.2019.10847. Epub 2019 Sep 10. Oncol Lett. 2019. PMID: 31611974 Free PMC article.
-
Cyclin F Downregulation Affects Epithelial-Mesenchymal Transition Increasing Proliferation and Migration of the A-375 Melanoma Cell Line.Cancer Manag Res. 2020 Dec 22;12:13085-13097. doi: 10.2147/CMAR.S279169. eCollection 2020. Cancer Manag Res. 2020. PMID: 33376401 Free PMC article.
-
Cellular plasticity and metastasis in breast cancer: a pre- and post-malignant problem.J Cancer Metastasis Treat. 2019;5:47. doi: 10.20517/2394-4722.2019.26. Epub 2019 Jun 13. J Cancer Metastasis Treat. 2019. PMID: 32355893 Free PMC article.
-
Cyclin A2 maintains colon homeostasis and is a prognostic factor in colorectal cancer.J Clin Invest. 2021 Feb 15;131(4):e131517. doi: 10.1172/JCI131517. J Clin Invest. 2021. PMID: 33332285 Free PMC article.
-
Unreported intrinsic disorder in proteins: Disorder emergency room.Intrinsically Disord Proteins. 2015 Apr 22;3(1):e1010999. doi: 10.1080/21690707.2015.1010999. eCollection 2015. Intrinsically Disord Proteins. 2015. PMID: 28232885 Free PMC article.
References
-
- Aaltomaa S, Lipponen P, Ala-Opas M, Eskelinen M, Syrjanen K, Kosma VM. Expression of cyclins A and D and p21(waf1/cip1) proteins in renal cell cancer and their relation to clinicopathological variables and patient survival. Br J Cancer. 1999;80:2001–2007. doi: 10.1038/sj.bjc.6690634. - DOI - PMC - PubMed
-
- Arpaia E, Blaser H, Quintela-Fandino M, Duncan G, Leong HS, Ablack A, Nambiar SC, Lind EF, Silvester J, Fleming CK, Rufini A, Tusche MW, Brustle A, Ohashi PS, Lewis JD, Mak TW. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk. Oncogene. 2012;31:884–896. doi: 10.1038/onc.2011.288. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

