Recombinant TLR5 agonist CBLB502 promotes NK cell-mediated anti-CMV immunity in mice
- PMID: 24879439
- PMCID: PMC4039429
- DOI: 10.1371/journal.pone.0096165
Recombinant TLR5 agonist CBLB502 promotes NK cell-mediated anti-CMV immunity in mice
Abstract
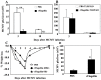
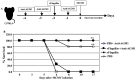
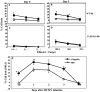
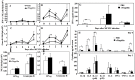
Prior work using allogeneic bone marrow transplantation (allo-BMT) models showed that peritransplant administration of flagellin, a toll-like receptor 5 (TLR5) agonist protected murine allo-BMT recipients from CMV infection while limiting graft-vs-host disease (GvHD). However, the mechanism by which flagellin-TLR5 interaction promotes anti-CMV immunity was not defined. Here, we investigated the anti-CMV immunity of NK cells in C57BL/6 (B6) mice treated with a highly purified cGMP grade recombinant flagellin variant CBLB502 (rflagellin) followed by murine CMV (mCMV) infection. A single dose of rflagellin administered to mice between 48 to 72 hours prior to MCMV infection resulted in optimal protection from mCMV lethality. Anti-mCMV immunity in rflagellin-treated mice correlated with a significantly reduced liver viral load and increased numbers of Ly49H+ and Ly49D+ activated cytotoxic NK cells. Additionally, the increased anti-mCMV immunity of NK cells was directly correlated with increased numbers of IFN-γ, granzyme B- and CD107a producing NK cells following mCMV infection. rFlagellin-induced anti-mCMV immunity was TLR5-dependent as rflagellin-treated TLR5 KO mice had ∼10-fold increased liver viral load compared with rflagellin-treated WT B6 mice. However, the increased anti-mCMV immunity of NK cells in rflagellin-treated mice is regulated indirectly as mouse NK cells do not express TLR5. Collectively, these data suggest that rflagellin treatment indirectly leads to activation of NK cells, which may be an important adjunct benefit of administering rflagellin in allo-BMT recipients.
Conflict of interest statement
Figures




Similar articles
-
The role of LY49 NK cell subsets in the regulation of murine cytomegalovirus infections.J Immunol. 1999 Jan 15;162(2):718-26. J Immunol. 1999. PMID: 9916691
-
Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity.J Immunol. 2011 Nov 15;187(10):5130-40. doi: 10.4049/jimmunol.1101334. Epub 2011 Oct 17. J Immunol. 2011. PMID: 22013117 Free PMC article.
-
Natural killer cell dependent within-host competition arises during multiple MCMV infection: consequences for viral transmission and evolution.PLoS Pathog. 2013 Jan;9(1):e1003111. doi: 10.1371/journal.ppat.1003111. Epub 2013 Jan 3. PLoS Pathog. 2013. PMID: 23300458 Free PMC article.
-
The impact of Ly49-NK cell-dependent recognition of MCMV infection on innate and adaptive immune responses.J Biomed Biotechnol. 2011;2011:641702. doi: 10.1155/2011/641702. Epub 2011 May 22. J Biomed Biotechnol. 2011. PMID: 21660138 Free PMC article. Review.
-
Genetic control of innate immune responses against cytomegalovirus: MCMV meets its match.Genes Immun. 2002 Aug;3(5):250-62. doi: 10.1038/sj.gene.6363876. Genes Immun. 2002. PMID: 12140743 Review.
Cited by
-
Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities.Pharmaceutics. 2022 Aug 11;14(8):1671. doi: 10.3390/pharmaceutics14081671. Pharmaceutics. 2022. PMID: 36015297 Free PMC article. Review.
-
Psychopharmacology: neuroimmune signaling in psychiatric disease-developing vaccines against abused drugs using toll-like receptor agonists.Psychopharmacology (Berl). 2019 Oct;236(10):2899-2907. doi: 10.1007/s00213-019-5176-9. Epub 2019 Feb 6. Psychopharmacology (Berl). 2019. PMID: 30726515 Review.
-
Modeling DNA damage-induced pneumopathy in mice: insight from danger signaling cascades.Radiat Oncol. 2017 Aug 24;12(1):142. doi: 10.1186/s13014-017-0865-1. Radiat Oncol. 2017. PMID: 28836991 Free PMC article. Review.
-
A deimmunized and pharmacologically optimized Toll-like receptor 5 agonist for therapeutic applications.Commun Biol. 2021 Apr 12;4(1):466. doi: 10.1038/s42003-021-01978-6. Commun Biol. 2021. PMID: 33846531 Free PMC article.
-
Flagellin engineering enhances CAR-T cell function by reshaping tumor microenvironment in solid tumors.J Immunother Cancer. 2025 Apr 5;13(4):e010237. doi: 10.1136/jitc-2024-010237. J Immunother Cancer. 2025. PMID: 40187752 Free PMC article.
References
-
- Paar DP, Pollard RB (1996) Immunotherapy of CMV infections. Advances in experimental medicine and biology 394: 145–151. - PubMed
-
- Nomura F, Shimokata K, Sakai S, Yamauchi T, Kodera Y, et al. (1990) Cytomegalovirus pneumonitis occurring after allogeneic bone marrow transplantation: a study of 106 recipients. Japanese journal of medicine 29: 595–602. - PubMed
-
- Langston AA, Redei I, Caliendo AM, Somani J, Hutcherson D, et al. (2002) Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood 99: 1085–1088. - PubMed
-
- Lenac T, Arapovic J, Traven L, Krmpotic A, Jonjic S (2008) Murine cytomegalovirus regulation of NKG2D ligands. Med Microbiol Immunol 197: 159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

