Marennine, promising blue pigments from a widespread Haslea diatom species complex
- PMID: 24879542
- PMCID: PMC4071570
- DOI: 10.3390/md12063161
Marennine, promising blue pigments from a widespread Haslea diatom species complex
Abstract
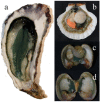
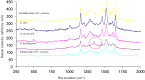
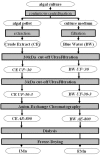
In diatoms, the main photosynthetic pigments are chlorophylls a and c, fucoxanthin, diadinoxanthin and diatoxanthin. The marine pennate diatom Haslea ostrearia has long been known for producing, in addition to these generic pigments, a water-soluble blue pigment, marennine. This pigment, responsible for the greening of oysters in western France, presents different biological activities: allelopathic, antioxidant, antibacterial, antiviral, and growth-inhibiting. A method to extract and purify marennine has been developed, but its chemical structure could hitherto not be resolved. For decades, H. ostrearia was the only organism known to produce marennine, and can be found worldwide. Our knowledge about H. ostrearia-like diatom biodiversity has recently been extended with the discovery of several new species of blue diatoms, the recently described H. karadagensis, H. silbo sp. inedit. and H. provincialis sp. inedit. These blue diatoms produce different marennine-like pigments, which belong to the same chemical family and present similar biological activities. Aside from being a potential source of natural blue pigments, H. ostrearia-like diatoms thus present a commercial potential for aquaculture, cosmetics, food and health industries.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

