Experimental and theoretical perspectives of the Noyori-Ikariya asymmetric transfer hydrogenation of imines
- PMID: 24879612
- PMCID: PMC6272002
- DOI: 10.3390/molecules19066987
Experimental and theoretical perspectives of the Noyori-Ikariya asymmetric transfer hydrogenation of imines
Abstract
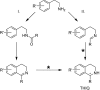
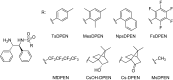
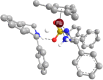
The asymmetric transfer hydrogenation (ATH) of imines catalyzed by the Noyori-Ikariya [RuCl(η6-arene)(N-arylsulfonyl-DPEN)] (DPEN=1,2-diphenylethylene-1,2-diamine) half-sandwich complexes is a research topic that is still being intensively developed. This article focuses on selected aspects of this catalytic system. First, a great deal of attention is devoted to the N-arylsulfonyl moiety of the catalysts in terms of its interaction with protonated imines (substrates) and amines (components of the hydrogen-donor mixture). The second part is oriented toward the role of the η6-coordinated arene. The final part concerns the imine substrate structural modifications and their importance in connection with ATH. Throughout the text, the summary of known findings is complemented with newly-presented ones, which have been approached both experimentally and computationally.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






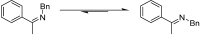
Similar articles
-
Opportunities offered by chiral η⁶-arene/N-arylsulfonyl-diamine-RuII catalysts in the asymmetric transfer hydrogenation of ketones and imines.Molecules. 2011 Jun 28;16(7):5460-95. doi: 10.3390/molecules16075460. Molecules. 2011. PMID: 21712760 Free PMC article. Review.
-
Practical aspects and mechanism of asymmetric hydrogenation with chiral half-sandwich complexes.Molecules. 2013 Jun 10;18(6):6804-28. doi: 10.3390/molecules18066804. Molecules. 2013. PMID: 23752467 Free PMC article. Review.
-
Heterocycle-containing Noyori-Ikariya catalysts for asymmetric transfer hydrogenation of ketones.Dalton Trans. 2022 Sep 13;51(35):13462-13469. doi: 10.1039/d2dt02411j. Dalton Trans. 2022. PMID: 35994090
-
New insight into the role of a base in the mechanism of imine transfer hydrogenation on a Ru(II) half-sandwich complex.Dalton Trans. 2013 Apr 14;42(14):5174-82. doi: 10.1039/c3dt32733g. Dalton Trans. 2013. PMID: 23403772
-
Highly enantioselective hydrogenation of N-aryl imines derived from acetophenones by using Ru-pybox complexes under hydrogenation or transfer hydrogenation conditions in isopropanol.Chemistry. 2015 Jan 7;21(2):549-53. doi: 10.1002/chem.201405276. Epub 2014 Nov 20. Chemistry. 2015. PMID: 25413251
Cited by
-
Effect of Ligand Substituents on Spectroscopic and Catalytic Properties of Water-Compatible Cp*Ir-(pyridinylmethyl)sulfonamide-Based Transfer Hydrogenation Catalysts.Inorg Chem. 2024 Feb 26;63(8):3815-3823. doi: 10.1021/acs.inorgchem.3c04040. Epub 2024 Feb 11. Inorg Chem. 2024. PMID: 38343274 Free PMC article.
-
Bifunctional thiosquaramide catalyzed asymmetric reduction of dihydro-β-carbolines and enantioselective synthesis of (-)-coerulescine and (-)-horsfiline by oxidative rearrangement.RSC Adv. 2020 Oct 21;10(63):38672-38677. doi: 10.1039/d0ra07705d. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517527 Free PMC article.
-
Special Issue on Ruthenium Complexes.Molecules. 2017 Feb 8;22(2):255. doi: 10.3390/molecules22020255. Molecules. 2017. PMID: 28208737 Free PMC article.
-
Editorial of Special Issue Ruthenium Complex: The Expanding Chemistry of the Ruthenium Complexes.Molecules. 2015 Sep 18;20(9):17244-74. doi: 10.3390/molecules200917244. Molecules. 2015. PMID: 26393560 Free PMC article.
References
-
- Whaley W.M., Govindachari T.R. The Pictet-Spengler synthesis of tetrahydroisoquinolines and related compounds. In: Adams R., editor. Organic Reactions. Volume 6. John Wiley & Sons, Inc.; New York, NY, USA: 1951. pp. 151–190.
-
- Whaley W.M., Govindachari T.R. Preparation of 3,4-dihydroisoquinolines and related compounds by the Bischler-Napieralski reaction. In: Adams R., editor. Organic Reactions. Volume 6. John Wiley & Sons, Inc.; New York, NY, USA: 1951. pp. 74–150.
-
- Gawley R.E., Aubé J. Principles of Asymmetric Synthesis. 2nd ed. Elsevier; Oxford, UK: 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

