Hemin controls T cell polarization in sickle cell alloimmunization
- PMID: 24879794
- PMCID: PMC4068268
- DOI: 10.4049/jimmunol.1400105
Hemin controls T cell polarization in sickle cell alloimmunization
Abstract
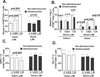
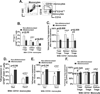
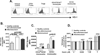
Patients with sickle cell disease (SCD) often require transfusions to treat and prevent worsening anemia and other SCD complications. However, transfusions can trigger alloimmunization against transfused RBCs with serious clinical sequelae. Risk factors for alloimmunization in SCD remain poorly understood. We recently reported altered regulatory T cell (Treg) and Th responses with higher circulating Th1 (IFN-γ(+)) cytokines in chronically transfused SCD patients with alloantibodies as compared with those without alloantibodies. Because monocytes play a critical role in polarization of T cell subsets and participate in clearance of transfused RBCs, we tested the hypothesis that in response to the RBC breakdown product hemin, monocyte control of T cell polarization will differ between alloimmunized and non-alloimmunized SCD patients. Exogenous hemin induced Treg polarization in purified T cell/monocyte cocultures from healthy volunteers through the monocyte anti-inflammatory heme-degrading enzyme heme oxygenase-1. Importantly, hemin primarily through its effect on CD16+ monocytes induced an anti-inflammatory (higher Treg/lower Th1) polarization state in the non-alloimmunized SCD group, whereas it had little effect in the alloimmunized group. Non-alloimmunized SCD CD16+ monocytes expressed higher basal levels of heme oxygenase-1. Furthermore, IL-12, which contributed to a proinflammatory polarization state (low Treg/high Th1) in SCD, was dampened in hemin-treated stimulated monocytes from non-alloimmunized SCD patients, but not in the alloimmunized group. These data suggest that unlike alloimmunized patients, non-alloimmunized SCD CD16+ monocytes in response to transfused RBC breakdown products promote an anti-inflammatory state that is less conducive to alloimmunization.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures


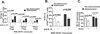
Similar articles
-
Immunoregulatory networks in sickle cell alloimmunization.Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):457-461. doi: 10.1182/asheducation-2016.1.457. Hematology Am Soc Hematol Educ Program. 2016. PMID: 27913516 Free PMC article. Review.
-
Mechanisms of sickle cell alloimmunization.Transfus Clin Biol. 2015 Aug;22(3):178-81. doi: 10.1016/j.tracli.2015.05.005. Epub 2015 Jun 6. Transfus Clin Biol. 2015. PMID: 26056038 Free PMC article. Review.
-
Altered heme-mediated modulation of dendritic cell function in sickle cell alloimmunization.Haematologica. 2016 Sep;101(9):1028-38. doi: 10.3324/haematol.2016.147181. Epub 2016 May 26. Haematologica. 2016. PMID: 27229712 Free PMC article.
-
Red blood cell alloimmunization is associated with lower expression of FcγR1 on monocyte subsets in patients with sickle cell disease.Transfusion. 2019 Oct;59(10):3219-3227. doi: 10.1111/trf.15463. Epub 2019 Jul 29. Transfusion. 2019. PMID: 31355970 Free PMC article.
-
Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease.Am J Hematol. 2013 Sep;88(9):736-40. doi: 10.1002/ajh.23488. Epub 2013 Jun 20. Am J Hematol. 2013. PMID: 23720018 Free PMC article.
Cited by
-
Immune Regulation of sickle Cell Alloimmunization.ISBT Sci Ser. 2017 Feb;12(1):248-253. doi: 10.1111/voxs.12296. Epub 2016 Nov 15. ISBT Sci Ser. 2017. PMID: 28261322 Free PMC article.
-
Inflammatory Dendritic Cells Contribute to Regulate the Immune Response in Sickle Cell Disease.Front Immunol. 2021 Feb 4;11:617962. doi: 10.3389/fimmu.2020.617962. eCollection 2020. Front Immunol. 2021. PMID: 33613546 Free PMC article.
-
Medical and economic implications of strategies to prevent alloimmunization in sickle cell disease.Transfusion. 2017 Sep;57(9):2267-2276. doi: 10.1111/trf.14212. Epub 2017 Jun 26. Transfusion. 2017. PMID: 28653325 Free PMC article. Review.
-
Type I interferon is induced by hemolysis and drives antibody-mediated erythrophagocytosis in sickle cell disease.Blood. 2021 Sep 30;138(13):1162-1171. doi: 10.1182/blood.2021011629. Blood. 2021. PMID: 34166491 Free PMC article.
-
Dynamics of antibody engagement of red blood cells in vivo and in vitro.Front Immunol. 2024 Nov 28;15:1475470. doi: 10.3389/fimmu.2024.1475470. eCollection 2024. Front Immunol. 2024. PMID: 39669570 Free PMC article.
References
-
- Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus. Med. Rev. 2007;21:118–133. - PubMed
-
- Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

