Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials
- PMID: 24879819
- PMCID: PMC4039388
- DOI: 10.1136/bmj.g3429
Time to treatment with recombinant tissue plasminogen activator and outcome of stroke in clinical practice: retrospective analysis of hospital quality assurance data with comparison with results from randomised clinical trials
Abstract
Objective: To study the time dependent effectiveness of thrombolytic therapy for acute ischaemic stroke in daily clinical practice.
Design: A retrospective cohort study using data from a large scale, comprehensive population based state-wide stroke registry in Germany.
Setting: All 148 hospitals involved in acute stroke care in a large state in southwest Germany with 10.4 million inhabitants.
Participants: Data from 84,439 patients with acute ischaemic stroke were analysed, 10,263 (12%) were treated with thrombolytic therapy and 74,176 (88%) were not treated.
Main outcome measures: Primary endpoint was the dichotomised score on a modified Rankin scale at discharge ("favourable outcome" score 0 or 1 or "unfavourable outcome" score 2-6) analysed by binary logistic regression. Patients treated with recombinant tissue plasminogen activator (rtPA) were categorised according to time from onset of stroke to treatment. Analogous analyses were conducted for the association between rtPA treatment of stroke and in-hospital mortality. As a co-primary endpoint the chance of a lower modified Rankin scale score at discharge was analysed by ordinal logistic regression analysis (shift analysis).
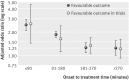
Results: After adjustment for characteristics of patients, hospitals, and treatment, rtPA was associated with better outcome in a time dependent pattern. The number needed to treat ranged from 4.5 (within first 1.5 hours after onset; odds ratio 2.49) to 18.0 (up to 4.5 hours; odds ratio 1.26), while mortality did not vary up to 4.5 hours. Patients treated with rtPA beyond 4.5 hours (including mismatch based approaches) showed a significantly better outcome only in dichotomised analysis (odds ratio 1.25, 95% confidence interval 1.01 to 1.55) but the mortality risk was higher (1.45, 1.08 to 1.92).
Conclusion: The effectiveness of thrombolytic therapy in daily clinical practice might be comparable with the effectiveness shown in randomised clinical trials and pooled analysis. Early treatment was associated with favourable outcome in daily clinical practice, which underlines the importance of speeding up the process for thrombolytic therapy in hospital and before admission to achieve shorter time from door to needle and from onset to treatment for thrombolytic therapy.
© Gumbinger et al 2014.
Conflict of interest statement
Competing interest: All authors have completed the ICMJE uniform disclosure form at
Figures



References
-
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-8. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317-29. - PubMed
-
- Hacke W, Donnan G, Fieschi C, Kaste M, Kummer R von, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768-74. - PubMed
-
- Lees KR, Bluhmki E, Kummer R von, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695-703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
