P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro
- PMID: 24879869
- PMCID: PMC4214662
- DOI: 10.1113/jphysiol.2013.268136
P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro
Abstract
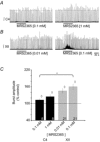
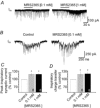
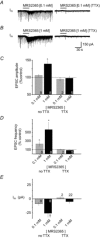
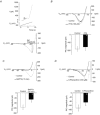
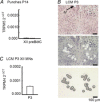
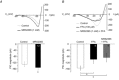
PreBötzinger complex inspiratory rhythm-generating networks are excited by metabotropic purinergic receptor subtype 1 (P2Y1R) activation. Despite this, and the fact that inspiratory MNs express P2Y1Rs, the role of P2Y1Rs in modulating motor output is not known for any MN pool. We used rhythmically active brainstem-spinal cord and medullary slice preparations from neonatal rats to investigate the effects of P2Y1R signalling on inspiratory output of phrenic and XII MNs that innervate diaphragm and airway muscles, respectively. MRS2365 (P2Y1R agonist, 0.1 mm) potentiated XII inspiratory burst amplitude by 60 ± 9%; 10-fold higher concentrations potentiated C4 burst amplitude by 25 ± 7%. In whole-cell voltage-clamped XII MNs, MRS2365 evoked small inward currents and potentiated spontaneous EPSCs and inspiratory synaptic currents, but these effects were absent in TTX at resting membrane potential. Voltage ramps revealed a persistent inward current (PIC) that was attenuated by: flufenamic acid (FFA), a blocker of the Ca(2+)-dependent non-selective cation current ICAN; high intracellular concentrations of BAPTA, which buffers Ca(2+) increases necessary for activation of ICAN; and 9-phenanthrol, a selective blocker of TRPM4 channels (candidate for ICAN). Real-time PCR analysis of mRNA extracted from XII punches and laser-microdissected XII MNs revealed the transcript for TRPM4. MRS2365 potentiated the PIC and this potentiation was blocked by FFA, which also blocked the MRS2365 potentiation of glutamate currents. These data suggest that XII MNs are more sensitive to P2Y1R modulation than phrenic MNs and that the P2Y1R potentiation of inspiratory output occurs in part via potentiation of TRPM4-mediated ICAN, which amplifies inspiratory inputs.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures





References
-
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. - PubMed
-
- Adachi T, Huxtable AG, Fang X, Funk GD. Substance P modulation of hypoglossal motoneuron excitability during development: changing balance between conductances. J Neurophysiol. 2010;104:854–872. - PubMed
-
- Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve α2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol. 2005;98:1297–1308. - PubMed
-
- Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. - PubMed
-
- Aoyama T, Koga S, Nakatsuka T, Fujita T, Goto M, Kumamoto E. Excitation of rat spinal ventral horn neurons by purinergic P2X and P2Y receptor activation. Brain Res. 2010;1340:10–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

