Antibody induction versus corticosteroid induction for liver transplant recipients
- PMID: 24880007
- PMCID: PMC10577808
- DOI: 10.1002/14651858.CD010252.pub2
Antibody induction versus corticosteroid induction for liver transplant recipients
Abstract
Background: Liver transplantation is an established treatment option for end-stage liver failure. To date, no consensus has been reached on the use of immunosuppressive T-cell specific antibody induction compared with corticosteroid induction of immunosuppression after liver transplantation.
Objectives: To assess the benefits and harms of T-cell specific antibody induction versus corticosteroid induction for prevention of acute rejection in liver transplant recipients.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 30 September 2013 together with reference checking, citation searching, contact with trial authors and pharmaceutical companies to identify additional trials.
Selection criteria: We included all randomised clinical trials assessing immunosuppression with T-cell specific antibody induction versus corticosteroid induction in liver transplant recipients. Our inclusion criteria stated that participants within each included trial should have received the same maintenance immunosuppressive therapy.
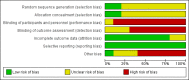
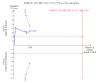
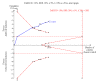
Data collection and analysis: We used RevMan for statistical analysis of dichotomous data with risk ratio (RR) and of continuous data with mean difference (MD), both with 95% confidence intervals (CIs). We assessed risk of systematic errors (bias) using bias risk domains with definitions. We used trial sequential analysis to control for random errors (play of chance).
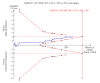
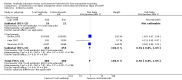
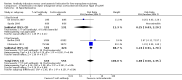
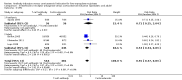
Main results: We included 10 randomised trials with a total of 1589 liver transplant recipients, which studied the use of T-cell specific antibody induction versus corticosteroid induction. All trials were with high risk of bias. We compared any kind of T-cell specific antibody induction versus corticosteroid induction in 10 trials with 1589 participants, including interleukin-2 receptor antagonist induction versus corticosteroid induction in nine trials with 1470 participants, and polyclonal T-cell specific antibody induction versus corticosteroid induction in one trial with 119 participants.Our analyses showed no significant differences regarding mortality (RR 1.01, 95% CI 0.72 to 1.43), graft loss (RR 1.12, 95% CI 0.82 to 1.53) and acute rejection (RR 0.84, 95% CI 0.70 to 1.00), infection (RR 0.96, 95% CI 0.85 to 1.09), hepatitis C virus recurrence (RR 0.89, 95% CI 0.79 to 1.00), malignancy (RR 0.59, 95% CI 0.13 to 2.73), and post-transplantation lymphoproliferative disorder (RR 1.00, 95% CI 0.07 to 15.38) when any kind of T-cell specific antibody induction was compared with corticosteroid induction (all low-quality evidence). Cytomegalovirus infection was less frequent in patients receiving any kind of T-cell specific antibody induction compared with corticosteroid induction (RR 0.50, 95% CI 0.33 to 0.75; low-quality evidence). This was also observed when interleukin-2 receptor antagonist induction was compared with corticosteroid induction (RR 0.55, 95% CI 0.37 to 0.83; low-quality evidence), and when polyclonal T-cell specific antibody induction was compared with corticosteroid induction (RR 0.21, 95% CI 0.06 to 0.70; low-quality evidence). However, when trial sequential analysis regarding cytomegalovirus infection was applied, the required information size was not reached. Furthermore, diabetes mellitus occurred less frequently when T-cell specific antibody induction was compared with corticosteroid induction (RR 0.45, 95% CI 0.34 to 0.60; low-quality evidence), when interleukin-2 receptor antagonist induction was compared with corticosteroid induction (RR 0.45, 95% CI 0.35 to 0.61; low-quality evidence), and when polyclonal T-cell specific antibody induction was compared with corticosteroid induction (RR 0.12, 95% CI 0.02 to 0.95; low-quality evidence). When trial sequential analysis was applied, the trial sequential monitoring boundary for benefit was crossed. We found no subgroup differences for type of interleukin-2 receptor antagonist (basiliximab versus daclizumab). Four trials reported on adverse events. However, no differences between trial groups were noted. Limited data were available for meta-analysis on drug-specific adverse events such as haematological adverse events for antithymocyte globulin. No data were available on quality of life.
Authors' conclusions: Because of the low quality of the evidence, the effects of T-cell antibody induction remain uncertain. T-cell specific antibody induction seems to reduce diabetes mellitus and may reduce cytomegalovirus infection when compared with corticosteroid induction. No other clear benefits or harms were associated with the use of T-cell specific antibody induction compared with corticosteroid induction. For some analyses, the number of trials investigating the use of T-cell specific antibody induction after liver transplantation is small, and the numbers of participants and outcomes in these randomised trials are limited. Furthermore, the included trials are heterogeneous in nature and have applied different types of T-cell specific antibody induction therapy. All trials were at high risk of bias. Hence, additional randomised clinical trials are needed to assess the benefits and harms of T-cell specific antibody induction compared with corticosteroid induction for liver transplant recipients. Such trials ought to be conducted with low risks of systematic error and of random error.
Conflict of interest statement
None known.
Figures

























































Update of
- doi: 10.1002/14651858.CD010252
Similar articles
-
Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients.Cochrane Database Syst Rev. 2018 Apr 9;4(4):CD007606. doi: 10.1002/14651858.CD007606.pub4. Cochrane Database Syst Rev. 2018. PMID: 29630730 Free PMC article.
-
Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients.Cochrane Database Syst Rev. 2014 Jun 5;2014(6):CD010253. doi: 10.1002/14651858.CD010253.pub2. Cochrane Database Syst Rev. 2014. PMID: 24901467 Free PMC article.
-
Immunosuppressive T-cell antibody induction for heart transplant recipients.Cochrane Database Syst Rev. 2013 Dec 2;2013(12):CD008842. doi: 10.1002/14651858.CD008842.pub2. Cochrane Database Syst Rev. 2013. PMID: 24297433 Free PMC article.
-
Antibody induction therapy for lung transplant recipients.Cochrane Database Syst Rev. 2013 Nov 27;2013(11):CD008927. doi: 10.1002/14651858.CD008927.pub2. Cochrane Database Syst Rev. 2013. PMID: 24282128 Free PMC article.
-
Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients.Cochrane Database Syst Rev. 2015 Dec 15;(12):CD007606. doi: 10.1002/14651858.CD007606.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Apr 09;4:CD007606. doi: 10.1002/14651858.CD007606.pub4. PMID: 26666504 Updated.
Cited by
-
Chronic rejection after liver transplantation: Opening the Pandora's box.World J Gastroenterol. 2021 Dec 7;27(45):7771-7783. doi: 10.3748/wjg.v27.i45.7771. World J Gastroenterol. 2021. PMID: 34963740 Free PMC article.
-
Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients.Cochrane Database Syst Rev. 2018 Apr 9;4(4):CD007606. doi: 10.1002/14651858.CD007606.pub4. Cochrane Database Syst Rev. 2018. PMID: 29630730 Free PMC article.
-
Review on immunosuppression in liver transplantation.World J Hepatol. 2015 Jun 8;7(10):1355-68. doi: 10.4254/wjh.v7.i10.1355. World J Hepatol. 2015. PMID: 26052381 Free PMC article. Review.
-
Maintenance immunosuppression for adults undergoing liver transplantation: a network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 31;3(3):CD011639. doi: 10.1002/14651858.CD011639.pub2. Cochrane Database Syst Rev. 2017. PMID: 28362060 Free PMC article.
-
Induction immunosuppression in adults undergoing liver transplantation: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 16;1(1):CD013203. doi: 10.1002/14651858.CD013203.pub2. Cochrane Database Syst Rev. 2020. PMID: 31978255 Free PMC article.
References
References to studies included in this review
Boillot 2005 {published data only}
-
- Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid‐free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transplantation 2005; Vol. 11, issue 1:61‐7. - PubMed
De Simone 2007 {published data only}
-
- Simone P, Carlis L, Filipponi F, Grazi GL, Cuomo O, Santaniello W, et al. Results of a multicenter, randomized open‐label, controlled clinical trial comparing basiliximab versus steroids in hepatitis C positive liver transplant patients. Transplant International 2007;20(Suppl 2):33.
-
- Simone P, Carlis L, Grazi GL, Cuomo O, Calise F, Castagneto M, et al. Results of a multicenter, randomized open‐label trial comparing basiliximab vs. steroids in HCV liver transplant patients. American Journal of Transplantation 2007;7(Suppl 2):312.
Eason 2003 {published data only}
-
- Eason JD, Blazek J, Mason A, Loss GE. Steroid‐free immunosuppression trough thymoglobulin induction in liver transplantation: results of a prospective randomized trial. Hepatology (Baltimore, Md.) 2000; Vol. 32, issue 4:208A.
-
- Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid‐free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transplantation 2001;7(8):693‐7. - PubMed
-
- Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE Jr. Steroid‐free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation 2003;75(8):1396‐9. - PubMed
-
- Nair S, Loss GE, Cohen AJ, Eason JD. Induction with rabbit antithymocyte globulin versus induction with corticosteroids in liver transplantation: Impact on recurrent hepatitis C virus infection. Transplantation 2006;81:620‐2. - PubMed
Kato 2001 {published data only}
-
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84:829‐35. - PubMed
-
- Kato T, Neff GW, Montalbano M, Hung O, Lavandera R, Levi D, et al. Steroid‐free induction with daclizumab and tacrolimus in liver transplant recipients with hepatitis C—A preliminary report (abstract). Hepatology 2001;34:362A.
Kato 2007 {published data only}
-
- Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid‐free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84:829‐35. - PubMed
-
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Hepatology 2005; Vol. 37, issue 2:1217‐9. - PubMed
Klintmalm 2011 {published data only}
-
- Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, et al. A randomized, multicenter study comparing steroid‐free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transplantation 2011;17(12):1394‐403. - PubMed
-
- Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, et al. Corticosteroid‐free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1‐year interim results of the HCV‐3 study. Liver Transplantation 2007;13(11):1521‐31. - PubMed
Lupo 2008 {published data only}
-
- Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation 2008;86(7):925‐31. - PubMed
-
- Lupo L, Ricci P, Caputi L, Tandoi F, Aquilino F, Palma G, et al. Basiliximab vs steroids in liver transplantation immunosuppression. A prospective randomized clinical trial. Liver Transplantation 2005;11(7):LB17.
Neumann 2012 {published data only}
Spada 2006 {published data only}
-
- Spada M, Bertani A, Petz W, Torri E, Sonzogni A, Guizzetti M, et al. A randomized trial for tacrolimus and steroids vs. tacrolimus and basiliximab in pediatric liver transplantation. Hepatology 2004; Vol. 40, issue 4 Suppl 1:473A.
-
- Spada M, Petz W, Bertani A, Riva S, Sonzogni A, Giovannelli M, et al. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. American Journal of Transplantation 2006; Vol. 6, issue 8:1913‐21. - PubMed
Washburn 2001 {published data only}
-
- Washburn K, Speeg KV, Esterl R, Cigarroa F, Pollack M, Tourtellot C, et al. Steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus, and mycophenolate mofetil. Transplantation 2001;72(10):1675‐9. - PubMed
References to studies excluded from this review
Asensio 2002 {published data only}
-
- Asensio M, Margarit C, Chavez R, Ortega J, Charco R, Iglesias J. Induction with basiliximab reduces acute rejection in pediatric liver transplant patients treated with tacrolimus and steroids. Transplantation Proceedings 2002;34:1970‐1. - PubMed
Becker 2008 {published data only}
-
- Becker T, Foltys D, Bilbao I, D'Amico D, Colledan M, Bernardos A, et al. Patient outcomes in two steroid‐free regimens using tacrolimus monotherapy after daclizumab induction and tacrolimus with mycophenolate mofetil in liver transplantation. Transplantation 2008;86(12):1689‐94. - PubMed
Benitez 2010 {published data only}
-
- Benitez CE, Puig‐Pey I, Lopez M, Martinez‐Llordella M, Lozano JJ, Bohne F, et al. ATG‐Fresenius treatment and low‐dose tacrolimus: results of a randomized controlled trial in liver transplantation. American Journal of Transplantation 2010;10:2296‐304. - PubMed
Bogetti 2005 {published data only}
-
- Bogetti D, Jarzembowski T‐M, Sankary H‐N, Manzelli A, Knight P‐S, Chejfec G, et al. Hepatic ischemia/reperfusion injury can be modulated with thymoglobulin induction therapy. Transplantation Proceedings 2005; Vol. 37, issue 1:404‐6. - PubMed
-
- Bogetti D, Sankary HN, Chejfec G, Cotler S, Testa G, Benedetti E. Thymoglobulin (TG) induction protects liver allografts from ischemia/reperfusion injury (IRI). American Journal of Transplantation 2004; Vol. 4, issue Suppl 8:25.
Boillot 2009 {published data only}
-
- Boillot O, Poncet G, Méchet I, Dumortier J, Delafosse B, Sagnard P, et al. Randomized trial of triple based immunosuppression using tacrolimus, mycophenolate mofetil and steroids quadruple regimen induction with thymoglobulin in liver transplantation. Hepatology 2000; Vol. 32, issue 4 (Pt 2):599A.
-
- Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, et al. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: a five‐year randomized prospective study. Liver Transplantation 2009;15(11):1426‐34. - PubMed
Calmus 2002 {published data only}
-
- Calmus Y, Scheele JR, Gonzalez‐Pinto I, Jaurrieta EJ, Klar E, Pageaux GP, et al. Immunoprophylaxis with basiliximab, a chimeric anti‐interleukin‐2 receptor monoclonal antibody, in combination with azathioprine‐containing triple therapy in liver transplant recipients. Liver Transplantation 2002;8(2):123‐31. - PubMed
Calmus 2010 {published data only}
-
- Calmus Y, Kamar N, Gugenheim J, Duvoux C, Ducerf C, Wolf P, et al. Assessing renal function with daclizumab induction and delayed tacrolimus introduction in liver transplant recipients. Transplantation 2010;89(12):1504‐10. - PubMed
Cosimi 1987 {published data only}
-
- Cosimi AB, Cho SI, Delmonico FL, Kaplan MM, Rohrer RJ, Jenkins RL. A randomized clinical trial comparing OKT3 and steroids for treatment of hepatic allograft rejection. Transplantation 1987; Vol. 43, issue 1:91‐5. - PubMed
Cosimi 1990 {published data only}
-
- Cosimi AB, Jenkins RL, Rohrer RJ, Delmonico FL, Hoffman M, Monaco AP. A randomized clinical trial of prophylactic OKT3 monoclonal antibody in liver allograft recipients. Archives of Surgery 1990; Vol. 125, issue 6:781‐4. - PubMed
Eason 2001 {published data only}
-
- Eason JD, Blazek J, Mason A, Nair S, Loss GE. Steroid‐free immunosuppression through thymoglobulin induction in liver transplantation. Transplantation Proceedings 2001;33:1470‐1. - PubMed
Eghtesad 2011 {published data only}
-
- Eghtesad B, Forrest T, Fijiki M, Diago T, Hodgkinson P, Hashimoto K, et al. A pilot randomized controlled clinical trial of thymoglobulin (r‐ATG) induction with extended delay of calcineurin inhibitor therapy in liver transplantation ‐ Interim analysis. Liver Transplantation 2011;17:S85.
Elkashab 1994 {published data only}
-
- Elkashab M, Reizig M, Greig PD, Cameron R, Phillips MJ, Chung S, et al. Incidence and patterns of rejection using different induction therapies in liver transplant recipients. Transplantation Proceedings 1994;26:2669‐71. - PubMed
Farges 1994 {published data only}
-
- Farges O, Ericzon BG, Bresson Hadni S, Lynch SV, Hockerstedt K, Houssin D, et al. A randomized trial of OKT3‐based versus cyclosporine‐based immunoprophylaxis after liver transplantation. Long‐term results of a European and Australian multicenter study. Transplantation 1994; Vol. 58, issue 8:891‐8. - PubMed
-
- Farges O, Ericzon BG, Miguet JP, Lynch S, Hockerstedt K, Chapuis Y, et al. [Immunoprophylaxis with OKT3 after hepatic transplantation: results of a multicenter, prospective, controlled study]. Annales de Chirurgie 1993; Vol. 47, issue 7:675.
Fasola 2005 {published data only}
-
- Fasola CG, Smallwood G, Martinez E, Steiber A, Heffron TG. Daclizumab (DZB) induction in adult hepatitis C (HCV) liver transplant recipients (OLT): a single center experience with different antibody doses. Liver Transplantation 2005;11:C‐44.
Figueras 2006 {published data only}
-
- Figueras J, Prieto M, Bernardos A, Rimola A, Suarez F, Urbina JO, et al. Daclizumab induction and maintenance steroid‐free immunosuppression with mycophenolate mofetil and tacrolimus to prevent acute rejection of hepatic allografts. Transplant International 2006;19(8):641‐8. - PubMed
Filipponi 2004 {published data only}
-
- Filipponi F, Callea F, Salizzoni M, Grazi GL, Fassati LR, Rossi M, et al. Double‐blind comparison of hepatitis C histological recurrence rate in HCV+ liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation 2004;78(10):1488‐95. - PubMed
-
- Filipponi F, Salizzoni M, Grazi G, Pisati R, Ferrara R. Study of simulect‐based, steroid‐free immunosuppressive regimen in HCV+ de novo liver transplant patients: preliminary results. Transplantation Proceedings 2001;33(7‐8):3211‐2. - PubMed
Fischer 2000 {published data only}
-
- Fischer S, Hopkinson D, Cassivi SD, Saad S, Paul A. Initial experience of combined immunosuppressive induction therapy with polyclonal antithymocyte antibody, FK 506 (tacrolimus), and prednisolone in clinical liver transplantation. Transplantation Proceedings 2000;32(8):2817‐9. - PubMed
Foroncewicz 2009 {published data only}
-
- Foroncewicz B, Mucha K, Ryszkowska E, Ciszek M, Ziolkowski J, Porowski D, et al. Safety and efficacy of steroid‐free immunosuppression with tacrolimus and daclizumab in liver transplant recipients: 6‐year follow‐up in a single center. Transplantation Proceedings 2009;41(8):3103‐6. - PubMed
Ganschow 2001 {published data only}
-
- Ganschow R, Broering DC, Stuerenburg I, Rogiers X, Hellwege HH, Burdelski M. First experience with basiliximab in pediatric liver graft recipients. Pediatric Transplantation 2001;5(5):353‐8. - PubMed
-
- Ganschow R, Grabhorn E, Schulz A, Hugo A, Rogiers X, Burdelski M. Long‐term results of basiliximab induction immunosuppression in pediatric liver transplant recipients. Pediatric Transplantation 2005;9:741‐5. - PubMed
-
- Ganschow R, Lyons M, Grabhorn E, Venzke A, Broering DC, Rogiers X, et al. Experience with basiliximab in pediatric liver graft recipients. Transplantation Proceedings 2001;33:3606‐7. - PubMed
Ganschow 2005 {published data only}
-
- Ganschow R, Melter M, Wallot M, Schulz A, Pfister ED, Baumann U, et al. Maintained efficacy with steroid minimization after pediatric liver transplantation with basiliximab (SIMULECT (R)) induction therapy: a multicenter randomized 12‐month trial. Liver Transplantation 2005;11(7):78.
Heffron 2002 {published data only}
-
- Heffron TG, Smallwood GA, Pillen T, Davis L, Martinez E, Romero R, et al. Liver transplant induction trial of daclizumab to spare calcineurin inhibition. Transplantation Proceedings 2002;34(5):1514‐5. - PubMed
Ilmakunnas 2010 {published data only}
-
- Ilmakunnas M, Tukiainen E, Rouhiainen A, Rauvala H, Nordin A, Makisalo H, et al. Dacluzimab reduces ischemia/reperfusion injury in human liver transplantation. Scandinavian Transplant Society Meeting. 2010:38.
Kamar 2004 {published data only}
-
- Kamar N, Ribes D, Sandres‐Saune K, Suc B, Barange K, Cointault O, et al. Efficacy and safety of induction therapy with rabbit antithymocyte globulins in liver transplantation for hepatitis C. Transplantation Proceedings 2004;39(6):2757‐61. - PubMed
Koch 2002 {published data only}
-
- Koch M, Niemeyer G, Patel I, Light S, Nashan B. Pharmacokinetics, pharmacodynamics, and immunodynamics of daclizumab in a two‐dose regimen in liver transplantation. Transplantation 2002;73(10):1640‐6. - PubMed
Langrehr 1997 {published data only}
-
- Bechstein WO, Langrehr JM, Lohmann R, Lobeck H, Blumhardt G, Neuhaus P. Interleukin‐2 receptor antibody versus ATG for induction immunosuppression after liver transplantation: results of a prospective randomized trial. Langenbecks Archiv Chirurgie 1994;379:99‐102.
-
- Langrehr J, Guckelberger O, Neumann U, Nussler N, Lobeck H, Lohmann R, et al. A randomized trial comparing quadruple induction therapy with ANTI‐IL‐2 receptor antibody or ATG after liver transplantation. www.astp.org 1997 (accessed September 2013):103.
-
- Langrehr JM, Guckelberger O, Bechstein WO, Lobeck H, Meuer S, Schlag H, et al. Quadruple induction therapy following liver transplantation with interleukin‐2 receptor antibody BT563 or ATG. A prospective randomized trial. Langenbecks Archiv fur.Chirurgie 1997;Suppl 1 Forumband:673‐6.
-
- Langrehr JM, Guckelberger O, Nussler N, Radtke A, Lemmens HP, Jonas S, et al. Interleukin‐2 receptor antibody versus antithymocyte globulin as part of quadruple induction therapy after orthotopic liver transplantation: a randomized study. Transplantation Proceedings 1996; Vol. 28, issue 6:3204. - PubMed
-
- Langrehr JM, Nussler NC, Neumann U, Guckelberger O, Lohmann R, Radtke A, et al. A prospective randomized trial comparing interleukin‐2 receptor antibody versus antithymocyte globulin as part of a quadruple immunosuppressive induction therapy following orthotopic liver transplantation. Transplantation 1997; Vol. 63, issue 12:1772‐81. - PubMed
Langrehr 1998 {published data only}
-
- Glanemann M, Langrehr JM, Raakow R, Guckelberger O, Lohmann R, Klupp J, et al. Anti‐IL‐2 receptor BT563 versus placebo: a randomized trial for induction therapy after liver transplantation. Transplantation Proceedings 1998;30(5):2159‐60. - PubMed
-
- Langrehr JM, Glanemann M, Guckelberger O, Klupp J, Neumann U, Machens C, et al. A randomized, placebo‐controlled trial with anti‐interleukin‐2 receptor antibody for immunosuppressive induction therapy after liver transplantation. Clinical Transplantation 1998;12(4):303‐12. - PubMed
-
- Langrehr JM, Glanemann M, Schneller A, Neumann U, Guckelberger O, Lohmann R, et al. A randomized trial comparing anti‐interleukin‐2 receptor antibody and placebo for immunosuppressive therapy after OLT. Transplantation Proceedings 1998; Vol. 30, issue 4:1445‐6. - PubMed
Langrehr 1998b {published data only}
-
- Langrehr JM, Schneller A, Guckelberger O, Lohmann R, Neumann U, Jonas S, et al. Comparision of quadruple induction including ATG or IL‐2R antibody with FK506‐based therapy after liver transplantation. Transplantation Proceedings 1998;30:1439‐40. - PubMed
Langrehr 2002 {published data only}
-
- Langrehr JM, Klupp J, Glanemann M, Junge G, Pfitzmann R, Bahra M, et al. Long‐term results of mycophenolate mofetil as part of immunosuppressive induction therapy after liver transplantation. Clinical Transplantation 2006;20(3):272‐83. - PubMed
-
- Langrehr JM, Neumann UP, Lang M, Möller AR, Jonas S, Settmacher U, et al. First results from a prospective randomized trial comparing steroid‐free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplantation Proceedings 2002;34:1565‐6. - PubMed
Lerut 2005 {published data only}
-
- Lerut J, Thuyne V, Mathijs J, Lemaire J, Talpe S, Roggen F, et al. Anti‐CD2 monoclonal antibody and tacrolimus in adult liver transplantation. Transplantation 2005;80:1186‐93. - PubMed
Lin 2005 {published data only}
-
- Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, et al. The renal‐sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transplantation 2005;11(10):1258‐64. - PubMed
Liu 2004 {published data only}
-
- Liu CL, Fan ST, Lo CM, Chan SC, Ng IO, Lai CL, et al. Interleukin‐2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transplantation 2004;10:728‐33. - PubMed
Mc Diarmid 1991 {published data only}
-
- McDiarmid SV, Busuttil RW, Levy P, Millis MJ, Terasaki PI, Ament ME. The long‐term outcome of OKT3 compared with cyclosporine prophylaxis after liver transplantation. Transplantation 1991; Vol. 52, issue 1:91‐7. - PubMed
-
- McDiarmid SV, Millis MJ, Terasaki P, Vargas JH, Ament ME, Busuttil W. Induction of immunosuppression in pediatric orthotopic liver transplantation. Clinical Transplantation 1991;5:174‐80.
-
- McDiarmid SV, Millis MJ, Terasaki PI, Ament ME, Busuttil RW. OKT3 prophylaxis in liver transplantation. Digestive Diseases and Sciences 1991; Vol. 36, issue 10:1418‐26. - PubMed
-
- Millis JM, McDiarmid SV, Hiatt JR, Brems JJ, Colonna JO 2nd, Klein AS, et al. Randomized prospective trial of OKT3 for early prophylaxis of rejection after liver transplantation. Transplantation 1989; Vol. 47, issue 1:82‐8. - PubMed
McVicar 1997 {published data only}
-
- McVicar JP, Kowdley K, Emond MJ, Barr D, Marsh CL, Carithers RL, et al. Induction immunosuppressive therapy is associated with a low rejection rate after liver transplantation. Clinical Transplantation 1997;11(4):328‐33. - PubMed
Nashan 1996 {published data only}
-
- Nashan B, Schlitt HJ, Schwinzer R, Ringe B, Kuse E, Tusch G, et al. Immunoprophylaxis with a monoclonal anti‐IL‐2 receptor antibody in liver transplant patients. Transplantation 1996; Vol. 61, issue 4:546‐54. - PubMed
-
- Nashan B, Schwinzer R, Schlitt HJ, Wonigeit K, Pichlmayr R. Immunological effects of the anti‐IL‐2 receptor monoclonal antibody BT 563 in liver allografted patients. Transplant Immunology 1995;3:203‐11. - PubMed
Neuberger 2009 {published data only}
-
- Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced‐dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. American Journal of Transplantation 2009;9(2):327‐36. - PubMed
Neuhaus 1993 {published data only}
-
- Neuhaus P, Bechstein WO, Blumhardt G, Wiens M, Lemmens P, Langrehr JM, et al. Comparison of quadruple immunosuppression after liver transplantation with ATG or IL‐2 receptor antibody. Transplantation 1993;55:1320‐7. - PubMed
Neuhaus 1995 {published data only}
-
- Jonas S, Guckelberger O, Muller AR, Langrehr JM, Settmacher U, Tullius SG, et al. Cyclosporine‐based quadruple induction therapy versus tacrolimus‐based dual immunosuppression after liver transplantation: ten‐year follow‐up. Transplantation Proceedings 2002;34(5):1504‐6. - PubMed
-
- Jonas S, Guckelberger O, Tullius SG, Steinmuller T, Muller AR, Grauhan O, et al. Corticosteroid‐free therapy after tacrolimus‐based dual immunosuppression versus cyclosporine‐based quadruple‐induction therapy. Transplantation Proceedings 2001;33(3):2232‐3. - PubMed
-
- Jonas S, Rayes N, Neumann U, Neuhaus R, Bechstein WO, Guckelberger O, et al. De novo malignancies after liver transplantation using tacrolimus‐based protocols or cyclosporine‐based quadruple immunosuppression with an interleukin‐2 receptor antibody or antithymocyte globulin. Cancer 1997;80(6):1141‐50. - PubMed
-
- Neuhaus P, Blumhardt G, Bechstein WO, Platz KP, Jonas S, Mueller AR, et al. Comparison of FK506‐ and cyclosporine‐based immunosuppression in primary orthotopic liver transplantation. Transplantation 1995;59(1):31‐40. - PubMed
Neuhaus 1997 {published data only}
-
- Neuhaus P, Langrehr JM, Williams R, Calne RY, Pichlmayr R, McMaster P. Tacrolimus‐based immunosuppression after liver transplantation: a randomised study comparing dual versus triple low‐dose oral regimens. Transplant International 1997;10:253‐61. - PubMed
Neuhaus 2000 {published data only}
-
- Klupp J, Bechstein WO, Pratschke J, Tullius SG, Gebhard A, Lobeck H, et al. Risk and benefit of antibody induction therapy in combination with tacrolimus immunosuppression after liver transplantation. Transplantation Proceedings 1998;30(4):1443‐4. - PubMed
-
- Langrehr JM, Klupp J, Junge G, Jonas S, Neuhaus R, Bechstein WO, et al. Quadruple versus dual tacrolimus‐based induction after liver transplantation: a prospective, randomized trial. Transplantation Proceedings 2001; Vol. 33, issue 3:2330‐1. - PubMed
-
- Langrehr JM, Klupp J, Pfitzmann R, Neumann U, Lohmann R, Jonas S, et al. A prospective, randomized trial with quadruple versus dual tacrolimus‐based induction after liver transplantation. Transplantation Proceedings 2001;33:1520. - PubMed
-
- Neuhaus P, Klupp J, Langrehr JM, Neumann U, Gebhardt A, Pratschke J, et al. Quadruple tacrolimus‐based induction therapy including azathioprine and ALG does not significantly improve outcome after liver transplantation when compared with standard induction with tacrolimus and steroids: results of a prospective, randomized trial. Transplantation 2000; Vol. 69, issue 11:2343‐53. - PubMed
Neuhaus 2002 {published data only}
-
- Nashan B, Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, et al. [The mono‐clonal anti‐IL 2 receptor antibody Basiliximab reduces the incidence and degree of acute rejections after liver transplantation]. Zeitschrift Fur Gastroenterologie 2000;38:536.
-
- Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double‐blind randomized placebo‐controlled trial. Liver Transplantation 2002;8:132‐42. - PubMed
-
- Neuhaus P, Nashan B, Clavin PA, Kittur D, Salizzoni M, Rimola A, et al. Basiliximab (Simulect) reduces the rate and severity of acute rejection in adult liver transplant recipients [abstract]. Liver Transplantation 2000; Vol. 6, issue 3:C‐25.
Niemeyer 2002 {published data only}
-
- Niemeyer G, Koch M, Light S, Kuse ER, Nashan B. Long‐term safety, tolerability and efficacy of daclizumab (Zenapax((R)) in a two‐dose regimen in liver transplant recipients. American Journal of Transplantation 2002;2(5):454‐60. - PubMed
Otero 2009 {published data only}
-
- Otero A, Varo E, Urbina JO, Martin‐Vivaldi, R, Cuervas‐Mons V, Gonzalez‐Pinto I, et al. A prospective randomized open study in liver transplant recipients: daclizumab, mycophenolate mofetil, and tacrolimus versus tacrolimus and steroids. Liver Transplantation 2009;15(11):1542‐52. - PubMed
Otto 1996 {published data only}
-
- Otto G, Hofmann WJ, Gaweco AS, Seelos R, Herfarth C, Meuer S. Influence of the anti‐CD25 monoclonal antibody BT563 on clinical and biological rejection after orthotopic liver transplantation. Transplantation Proceedings 1996; Vol. 28, issue 6:3210‐1. - PubMed
Ramirez 2007 {published data only}
-
- Ramirez CB, Doria C, Francesco F, Iaria M, Kang YG, Marino IR. Anti‐IL2 induction in liver transplantation with 93% rejection‐free patient and graft survival at 18 months. Journal of Surgical Research 2007;138(2):198‐204. - PubMed
Reding 1996 {published data only}
-
- Reding R, Feyaerts A, Vraux H, Latinne D, De‐La Parra B, Cornet A, et al. Prophylactic immunosuppression with anti‐interleukin‐2 receptor monoclonal antibody LO‐Tact‐1 versus OKT3 in liver allografting. A two‐year follow‐up study. Transplantation 1996;61:1406‐9. - PubMed
-
- Reding R, Vraux H, Ville de Goyet J, Sokal E, Hemptinne B, Latinne D, et al. Monoclonal antibodies in prophylactic immunosuppression after liver transplantation. A randomized controlled trial comparing OKT3 and anti‐IL‐2 receptor monoclonal antibody LO‐Tact‐1. Transplantation 1993; Vol. 55, issue 3:534‐41. - PubMed
Schmeding 2007 {published data only}
-
- Schmeding M, Sauer IM, Kiessling A, Pratschke J, Neuhaus R, Neuhaus P, et al. Influence of basiliximab induction therapy on long term outcome after liver transplantation, a prospectively randomised trial. Annals of Transplantation 2007;12(3):15‐21. - PubMed
Schulak 1997 {published data only}
-
- Schulak J, May E, Post A, Fasola C, Mulligan D, Sterling R. Reduction of early rejection in adult liver transplantation with ATG induction therapy. Transplantation Proceedings 1997;29:555‐6. - PubMed
Schuller 2005 {published data only}
-
- Schuller S, Wiederkehr JC, Coelho Lemos I, Avilla SG, Schultz C. Daclizumab induction therapy associated with tacrolimus‐MMF has better outcome compared with tacrolimus‐MMF alone in pediatric living donor liver transplantation. Transplantation Proceedings 2005;37:1151‐2. - PubMed
Strehlau 2000 {published data only}
-
- Strehlau J, Pape L, Offner G, Nashan B, Ehrich JH. Interleukin‐2 receptor antibody‐induced alterations of ciclosporin dose requirements in paediatric transplant recipients. Lancet 2000;356:1327‐8. - PubMed
Yan 2004 {published data only}
-
- Yan LN, Wang WT, Li B, Lu SC, Wen TF. Induction with basiliximab reduces acute rejection in Chinese liver transplant recipients treated with cyclosporin, steroids and MMF. Liver Transplantation 2004;10:C‐5.
Yoshida 2005 {published data only}
-
- Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low‐dose tacrolimus regimen vs. a standard‐dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transplantation 2005;11(9):1064‐72. - PubMed
Additional references
Adams 1992
-
- Adams DH, Neuberger JM. Treatment of acute rejection. Seminars in Liver Disease 1992;12(1):80‐8. - PubMed
Banff 1997
-
- Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997; Vol. 25, issue 3:658‐63. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Chatenoud 2008
-
- Chatenoud L. The long and winding road towards induction of allograft tolerance in the clinic. Transplant International 2008;21(8):725‐7. - PubMed
Chen 2006
-
- Chen W, Zhang L. Regulatory T‐cell subsets and their roles in transplantation tolerance. Current Opinion in Organ Transplantation 2006;11(4):373‐8.
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 4 September 2012).
Dienstag 2012
-
- Dienstag JL, Cosimi AB. Liver transplantation — A vision realized. New England Journal of Medicine 2012;367(16):1483‐5. - PubMed
Egger 1997
FK506 1994a
-
- The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. New England Journal of Medicine 1994;331(17):1110‐5. - PubMed
FK506 1994b
-
- European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet 1994;344(8920):423‐8. - PubMed
Flechner 2008
-
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor–sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clinical Transplantation 2008;22(1):1‐15. - PubMed
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 12. Art. No.: LIVER.
Goralczyk 2011
-
- Goralczyk AD, Hauke N, Bari N, Tsui TY, Lorf T, Obed A. Interleukin 2 receptor antagonists for liver transplant recipients: a systematic review and meta‐analysis of controlled studies. Hepatology 2011;54(2):541‐54. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, Barnett International/PAREXEL, 1997.
Iversen 2009
-
- Iversen M, Corris P. Immunosuppression. In: Fisher AJ, Verleden G, Massard G editor(s). European Respiratory Monthly: Lung Transplantation. Plymouth, UK.: European Respiratory Society Journals Ltd., 2009:147‐68.
Keus 2009
-
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [MEDLINE: ] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lechler 2005
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation ‐ How much of the promise has been realized?. Nature Medicine 2005;11(6):605‐13. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Magliocca 2006
-
- Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath‐1H) for immunosuppressive therapy in organ transplantation. Transplant International 2006;19(9):705‐14. - PubMed
Marcos 2004
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
Moser 2002
-
- Moser MA. Options for induction immunosuppression in liver transplant recipients. Drugs 2002;62(7):995‐1011. - PubMed
O'Grady 2002
-
- O'Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002;360(9340):1119‐25. - PubMed
OPTN 2009
-
- OPTN/SRTR Annual Report. The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. www.ustransplant.org/annual_reports/current/ 2009 (accessed 30 May 2014).
Penninga 2012b
Penninga 2012c
Penninga 2013
Penninga 2013a
Perera 2009
-
- Perera MT, Mirza DF, Elias E. Liver transplantation: issues for the next 20 years. Journal of Gastroenterology and Hepatology 2009;24(Suppl 3):S124‐31. - PubMed
Pillai 2009
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Segev 2008
-
- Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. Steroid avoidance in liver transplantation: meta‐analysis and meta‐regression of randomized trials. Liver Transplantation 2008;14(4):512‐25. - PubMed
Seyfert‐Margolis 2010
-
- Seyfert‐Margolis V, Feng S. Tolerance: is it achievable in pediatric solid organ transplantation?. Pediatric Clinics of North America 2010;57:523‐38. - PubMed
Sgourakis 2009
-
- Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, et al. Corticosteroid‐free immunosuppression in liver transplantation: a meta‐analysis and meta‐regression of outcomes. Transplant International 2009;22(9):892‐905. - PubMed
Starzl 2003
Starzl 2008
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [MEDLINE: ] - PubMed
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
Transplant Activity Report 2009
-
- Transplant activity report 2008/2009 UK. www.organdonation.nhs.uk/ukt/statistics/statistics.jsp 2009 (accessed 30 May 2014).
TSA 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 April 2014).
Uemura 2011
-
- Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti‐thymocyte globulin, daclizumab, and corticosteroid. Transplant International 2011;24(7):640‐50. - PubMed
Wang 2010
-
- Wang XF, Li JD, Peng Y, Dai Y, Shi G, Xu W. Interleukin‐2 receptor antagonists in liver transplantation: a meta‐analysis of randomized trials. Transplantation Proceedings 2010;42(10):4567‐72. - PubMed
Webster 2010
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wiesner 2003
-
- Wiesner RH, Rakela J, Ishitani MB, Mulligan DC, Spivey JR, Steers JL, et al. Recent advances in liver transplantation. Mayo Clinic Proceedings 2003; Vol. 78, issue 2:197‐210. - PubMed
Wood 2008
Zaydfudim 2012
-
- Zaydfudim V, Feurer ID, Landman MP, Moore DE, Wright JK, Pinson CW. Reduction in corticosteroids is associated with better health‐related quality of life after liver transplantation. Journal of the American College of Surgeons 2012;214(2):164‐73. - PubMed
References to other published versions of this review
Penninga 2012a
-
- Penninga L, Wettergren A, Wilson CH, Steinbrüchel DA, Gluud C. Immunosuppressive T cell antibody induction therapy for liver transplant recipients. Cochrane Database of Systematic Reviews 2012, Issue 11, 2011 ‐ 10, 2012. [DOI: 10.1002/14651858.CD007341.pub2] - DOI
Wilson 2011
-
- Wilson CH, Asher JF, Manas DM. Immunosuppressive T cell antibodies for liver transplant recipients. Cochrane Database of Systematic Reviews 2011, Issue 3, 2008 ‐ 10, 2011. [DOI: 10.1002/14651858.CD007341] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

