Identification of distinct ChAT⁺ neurons and activity-dependent control of postnatal SVZ neurogenesis
- PMID: 24880216
- PMCID: PMC4122286
- DOI: 10.1038/nn.3734
Identification of distinct ChAT⁺ neurons and activity-dependent control of postnatal SVZ neurogenesis
Abstract
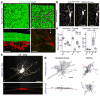
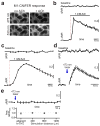
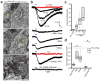
Postnatal and adult subventricular zone (SVZ) neurogenesis is believed to be primarily controlled by neural stem cell (NSC)-intrinsic mechanisms, interacting with extracellular and niche-driven cues. Although behavioral experiments and disease states have suggested possibilities for higher level inputs, it is unknown whether neural activity patterns from discrete circuits can directly regulate SVZ neurogenesis. We identified a previously unknown population of choline acetyltransferase (ChAT)(+) neurons residing in the rodent SVZ neurogenic niche. These neurons showed morphological and functional differences from neighboring striatal counterparts and released acetylcholine locally in an activity-dependent fashion. Optogenetic inhibition and stimulation of subependymal ChAT(+) neurons in vivo indicated that they were necessary and sufficient to control neurogenic proliferation. Furthermore, whole-cell recordings and biochemical experiments revealed direct SVZ NSC responses to local acetylcholine release, synergizing with fibroblast growth factor receptor activation to increase neuroblast production. These results reveal an unknown gateway connecting SVZ neurogenesis to neuronal activity-dependent control and suggest possibilities for modulating neuroregenerative capacities in health and disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
ChAT me up: how neurons control stem cells.Nat Neurosci. 2014 Jul;17(7):897-8. doi: 10.1038/nn.3746. Nat Neurosci. 2014. PMID: 24964158 No abstract available.
Similar articles
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
-
Cortical regulation of neurogenesis and cell proliferation in the ventral subventricular zone.Cell Rep. 2023 Jul 25;42(7):112783. doi: 10.1016/j.celrep.2023.112783. Epub 2023 Jul 8. Cell Rep. 2023. PMID: 37422764 Free PMC article.
-
ChAT-positive neurons participate in subventricular zone neurogenesis after middle cerebral artery occlusion in mice.Behav Brain Res. 2017 Jan 1;316:145-151. doi: 10.1016/j.bbr.2016.09.007. Epub 2016 Sep 5. Behav Brain Res. 2017. PMID: 27609645 Free PMC article.
-
Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice.Neurobiol Dis. 2017 Feb;98:9-24. doi: 10.1016/j.nbd.2016.11.005. Epub 2016 Nov 21. Neurobiol Dis. 2017. PMID: 27884724 Free PMC article.
-
The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain.J Neural Transm (Vienna). 2009 Aug;116(8):995-1005. doi: 10.1007/s00702-009-0207-z. Epub 2009 Mar 17. J Neural Transm (Vienna). 2009. PMID: 19291360 Review.
Cited by
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
-
Neural stem cell niche heterogeneity.Semin Cell Dev Biol. 2019 Nov;95:42-53. doi: 10.1016/j.semcdb.2019.01.005. Epub 2019 Jan 14. Semin Cell Dev Biol. 2019. PMID: 30639325 Free PMC article. Review.
-
Sex Steroids and Adult Neurogenesis in the Ventricular-Subventricular Zone.Front Endocrinol (Lausanne). 2018 Apr 9;9:156. doi: 10.3389/fendo.2018.00156. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29686651 Free PMC article. Review.
-
Uncovering inherent cellular plasticity of multiciliated ependyma leading to ventricular wall transformation and hydrocephalus.Nat Commun. 2018 Apr 25;9(1):1655. doi: 10.1038/s41467-018-03812-w. Nat Commun. 2018. PMID: 29695808 Free PMC article.
-
Cortical regulation of neurogenesis and cell proliferation in the ventral subventricular zone.Cell Rep. 2023 Jul 25;42(7):112783. doi: 10.1016/j.celrep.2023.112783. Epub 2023 Jul 8. Cell Rep. 2023. PMID: 37422764 Free PMC article.
References
-
- Kelsch W, Sim S, Lois C. Watching synaptogenesis in the adult brain. Annu Rev Neurosci. 2010;33:131–149. - PubMed
-
- Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. - PubMed
-
- Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron. 2011;70:597–613. - PubMed
-
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

