OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds
- PMID: 24880337
- PMCID: PMC4153440
- DOI: 10.1093/mp/ssu067
OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds
Abstract
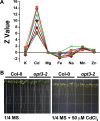
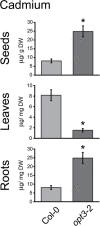
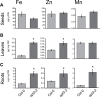
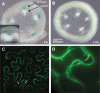
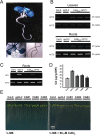
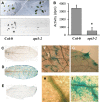
Plants and seeds are the main dietary sources of zinc, iron, manganese, and copper, but are also the main entry point for toxic elements such as cadmium into the food chain. We report here that an Arabidopsis oligopeptide transporter mutant, opt3-2, over-accumulates cadmium (Cd) in seeds and roots but, unexpectedly, under-accumulates Cd in leaves. The cadmium distribution in opt3-2 differs from iron, zinc, and manganese, suggesting a metal-specific mechanism for metal partitioning within the plant. The opt3-2 mutant constitutively up-regulates the Fe/Zn/Cd transporter IRT1 and FRO2 in roots, indicative of an iron-deficiency response. No genetic mutants that impair the shoot-to-root signaling of iron status in leaves have been identified. Interestingly, shoot-specific expression of OPT3 rescues the Cd sensitivity and complements the aberrant expression of IRT1 in opt3-2 roots, suggesting that OPT3 is required to relay the iron status from leaves to roots. OPT3 expression was found in the vasculature with preferential expression in the phloem at the plasma membrane. Using radioisotope experiments, we found that mobilization of Fe from leaves is severely affected in opt3-2, suggesting that Fe mobilization out of leaves is required for proper trace-metal homeostasis. When expressed in yeast, OPT3 does not localize to the plasma membrane, precluding the identification of the OPT3 substrate. Our in planta results show that OPT3 is important for leaf phloem-loading of iron and plays a key role regulating Fe, Zn, and Cd distribution within the plant. Furthermore, ferric chelate reductase activity analyses provide evidence that iron is not the sole signal transferred from leaves to roots in leaf iron status signaling.
Keywords: ionomics.; iron deficiency; metal homeostasis; phloem transport; seed loading.
© The Author 2014. Published by Oxford University Press on behalf of CSPB and IPPE, SIBS, CAS.
Figures

Similar articles
-
Changes in iron availability in Arabidopsis are rapidly sensed in the leaf vasculature and impaired sensing leads to opposite transcriptional programs in leaves and roots.Plant Cell Environ. 2018 Oct;41(10):2263-2276. doi: 10.1111/pce.13192. Epub 2018 Jun 19. Plant Cell Environ. 2018. PMID: 29520929
-
The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds.Plant Physiol. 2008 Feb;146(2):589-601. doi: 10.1104/pp.107.108183. Epub 2007 Dec 14. Plant Physiol. 2008. PMID: 18083798 Free PMC article.
-
Loss of OPT3 function decreases phloem copper levels and impairs crosstalk between copper and iron homeostasis and shoot-to-root signaling in Arabidopsis thaliana.Plant Cell. 2023 May 29;35(6):2157-2185. doi: 10.1093/plcell/koad053. Plant Cell. 2023. PMID: 36814393 Free PMC article.
-
Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification.Int J Mol Sci. 2015 Aug 13;16(8):19111-29. doi: 10.3390/ijms160819111. Int J Mol Sci. 2015. PMID: 26287170 Free PMC article. Review.
-
The zinc homeostasis network of land plants.Biochim Biophys Acta. 2012 Sep;1823(9):1553-67. doi: 10.1016/j.bbamcr.2012.05.016. Epub 2012 May 22. Biochim Biophys Acta. 2012. PMID: 22626733 Review.
Cited by
-
Post-Transcriptional Coordination of the Arabidopsis Iron Deficiency Response is Partially Dependent on the E3 Ligases RING DOMAIN LIGASE1 (RGLG1) and RING DOMAIN LIGASE2 (RGLG2).Mol Cell Proteomics. 2015 Oct;14(10):2733-52. doi: 10.1074/mcp.M115.048520. Epub 2015 Aug 7. Mol Cell Proteomics. 2015. PMID: 26253232 Free PMC article.
-
Transcriptome analysis of grapevine under salinity and identification of key genes responsible for salt tolerance.Funct Integr Genomics. 2019 Jan;19(1):61-73. doi: 10.1007/s10142-018-0628-6. Epub 2018 Jul 19. Funct Integr Genomics. 2019. PMID: 30046943
-
Iron-Nicotianamine Transporters Are Required for Proper Long Distance Iron Signaling.Plant Physiol. 2017 Nov;175(3):1254-1268. doi: 10.1104/pp.17.00821. Epub 2017 Sep 11. Plant Physiol. 2017. PMID: 28894019 Free PMC article.
-
An amiRNA screen uncovers redundant CBF and ERF34/35 transcription factors that differentially regulate arsenite and cadmium responses.Plant Cell Environ. 2021 May;44(5):1692-1706. doi: 10.1111/pce.14023. Epub 2021 Feb 25. Plant Cell Environ. 2021. PMID: 33554343 Free PMC article.
-
Common Bean: A Legume Model on the Rise for Unraveling Responses and Adaptations to Iron, Zinc, and Phosphate Deficiencies.Front Plant Sci. 2016 May 3;7:600. doi: 10.3389/fpls.2016.00600. eCollection 2016. Front Plant Sci. 2016. PMID: 27200068 Free PMC article. Review.
References
-
- Clemens S., Aarts M.G., Thomine S., Verbruggen N. (2013). Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci. 18, 92–99 - PubMed
-
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

