Identification of erythroferrone as an erythroid regulator of iron metabolism
- PMID: 24880340
- PMCID: PMC4104984
- DOI: 10.1038/ng.2996
Identification of erythroferrone as an erythroid regulator of iron metabolism
Erratum in
-
Author Correction: Identification of erythroferrone as an erythroid regulator of iron metabolism.Nat Genet. 2020 Apr;52(4):463. doi: 10.1038/s41588-019-0548-y. Nat Genet. 2020. PMID: 32107478
Abstract
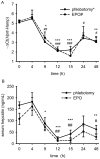
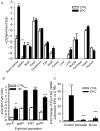
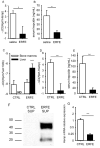
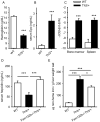
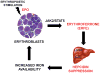
Recovery from blood loss requires a greatly enhanced supply of iron to support expanded erythropoiesis. After hemorrhage, suppression of the iron-regulatory hormone hepcidin allows increased iron absorption and mobilization from stores. We identified a new hormone, erythroferrone (ERFE), that mediates hepcidin suppression during stress erythropoiesis. ERFE is produced by erythroblasts in response to erythropoietin. ERFE-deficient mice fail to suppress hepcidin rapidly after hemorrhage and exhibit a delay in recovery from blood loss. ERFE expression is greatly increased in Hbb(th3/+) mice with thalassemia intermedia, where it contributes to the suppression of hepcidin and the systemic iron overload characteristic of this disease.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R01-DK-065029/DK/NIDDK NIH HHS/United States
- R01 DK090554/DK/NIDDK NIH HHS/United States
- CA-16042/CA/NCI NIH HHS/United States
- AI-28697/AI/NIAID NIH HHS/United States
- P30 DK041301/DK/NIDDK NIH HHS/United States
- P30-DK-041301/DK/NIDDK NIH HHS/United States
- R01 DK065029/DK/NIDDK NIH HHS/United States
- R01-DK-090554/DK/NIDDK NIH HHS/United States
- 5R01-DK-095112/DK/NIDDK NIH HHS/United States
- P30-CA-016042/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

